Bioen
599F., Autumn 2000
Bioengineering
Principles of Physiology
Bioeng 599F Final Examination
For questions, answers and comments on the final, go here: FinalExam&Ans.doc
Midterm Exam
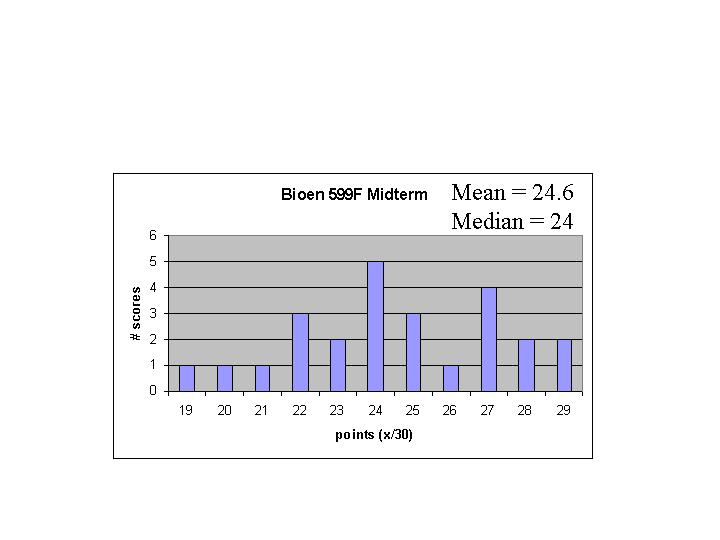
Bioen 599F - Mid-term Answers
Question 1.
A. The duty ratio is the fraction of the ATPase cycle time the motor is attached to the track along which it moves: ton/ttotal (actin for myosin; microtubules for kinesin). The duty ratio is much greater for kinesin (up to 0.5) compared with myosin (< 0.05 with up to 0.2 acceptable)
B. Myosin resides in a highly ordered structure and works in concert with many other motors in parallel to preform its task - pulling the thin filament towards the center of the sarcomere. A kinesin motor is processive, ie. can works alone or in small numbers. For kinesin to move down a microtubule it must always have one of its two heads on the microtubule; otherwise it could diffuse away. In contrast it is desirable for myosin to detach rapidly so that it does not impede (resist) the working stroke of other motors, ie. it works in a non-processive manner.
C. For a single attachment cycle, speed can be determined as V = Duni/ton where Duni is the unitary displacement or working stroke distance, and ton is the time the motor is on its track). ton is much longer for kinesin. Therefore one would expect V to be much slower for kinesin than myosin. If you are considering multiple cycles that would occur in an in vitro motility assay, speed would depend on ATPase rate. Since ton and ATPase usually co-vary, either justification is OK to answer this question.
Question 2.
A. Decrease - ATPase rate would decrease dramatically because the substrate concentration was reduced below the Km for the enzyme complex.
B. No change - Because actin sites freely available with purified filaments; there are no regulatory proteins present. OK if students say Ca2+ binds to regulatory light chain and increases slightly.
C. Decrease - Stop Now the thin filament is fully inhibited and myosin binding is prevented because Ca2+ is absent. There will be no actin-activated myosin ATPase
D. No Change - CK would not even catalyze a reaction because the assay mixture does not contain PCr or creatine.
QUESTION 3
A. Decreased - Less force at the longer length because the overlap of the thick and thin filaments is less and thus there are fewer cross bridges capable of generating force.
B. More - The person with the higher ATPase rate will have higher power, because everything else in the problem being the same, more chemical power input translates to more mechanical power output.
C. the muscle with the higher ATPase rate - The higher ATPase rate means a greater rate of cross bridge (myosin motor) turnover. At a fixed distance moved per myosin motor, this increased turnover rate is equivalent to increased shortening speed. Higher turnover rate means a greater intrinsic speed at the sarcomere level which translates in whole cells to greater speed of shortening if the load in the problem were the same.
QUESTION 4: Protein Supernatant fraction; Membrane fraction; Myofibrillar fraction
Myosin 0 0 X
actin 0 0 X
titin 0 0 X
Z line proteins 0 0 X
Mitochondrial ATP synthase 0 X 0
TCA cycle enzymes 0 X 0
Adenylate kinase X X ok is state mito bound 0
Enzymes for glycolysis X 0 0
Creatine kinase X X ok if state mito bound X ok if state myfibrillar bound
QUESTION 5 - Order the following events in their correct sequential order in muscle contraction and relaxation.
Binding of Ca2+ concentration to troponin 2
Increased myosin binding to actin 3
Increase in muscle oxygen consumption 5
Increase in Ca2+ in the cytoplasm 1
Development of force and/or shortening 4
QUESTION 6
Key features of the answer are: [PCr] > [ATP]; CK reaction flux is higher than ATPase and ATP synthase: CK reactions remain near equilibrium, buffers ATP. Net reaction is PCr to C + Pi by summary ATP to ADP + Pi & PCr + ADP to ATP + Cr . The simultaneous solution of both equations leads to little or no change in ATP or ADP.
QUESTION 7
For the metabolism of one 6 carbon glucose or glycosyl unit from glycogen: T: more ATP is produced starting from glycogen than from glucose
F: more ATP is produced in glycolysis than from TCA cycle and oxidative phosphorylation
F: there are both V and [H+] gradients
QUESTION 8.
Km is the [S] where the reaction velocity is ½ Vmax. The other key feature in drawing the curve is that Vmax will not be attained by 100 µM substrate. At 100 µM substrate the reaction velocity is ~500 µM/min or 0.83 Vmax.
QUESTION 9
Positive cooperativity -as the # of substrate molecules increases, the rate of the reaction will increase more than linearly - the curve shows a (slightly) sigmoidal shape Reaction is slow at low [S] - as [S] increases, the rate is increased - ie. the Hill coefficient n >1.
Last Updated:
11/01/00
Contact the instructor at: mregnier@u.washington.edu