
|
Denosumab is a new osteoporosis medication that was approved in June 2010 by the FDA for treatment of severe osteoporosis. This drug inhibits bone resorption. It is an antibody to RANK-Ligand, the factor made by osteoblasts that is necessary for the formation of mature osteoclasts. Here's how to remember the generic name:
The brand name is "Prolia"
Clinical trials have also suggested it is effective in rheumatoid arthritis and cancer with metastases to the bone.
On a physiological basis it would be a good choice for patients with any disease characterized by rapid bone resorption. I do not think it should be a first-line medication for most cases of osteoporosis.
The dose is 60mg given as sub-cutaneous injection once every 6 months. Adequate calcium and vitamin D are important while taking this medicine. In patients with cancer to the bone, the dose can be higher.
Bone pain and back pain were commonly reported but were not different from placebo-treated patients (35% with back pain, 12% with extremity pain, 4% with bone pain)
New cancers or opportunistic infections were not significantly higher than in placebo groups. However, there is theoretical concern about this because the RANK-RANK-ligand signalling system is used by other cells, particularly the white blood cells that fight infections and tumors. Therefore, denosumab should be used cautiously in patients at high risk for infection.
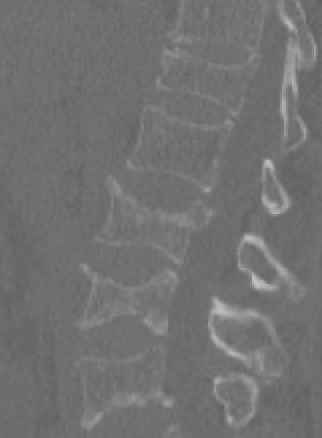
Multiple vertebral fractures if the dose is delayed
There are now more and more cases of multiple vertebral fractures that can occur within 2 months after a dose was skipped. Here is my 2023 editorial about this.
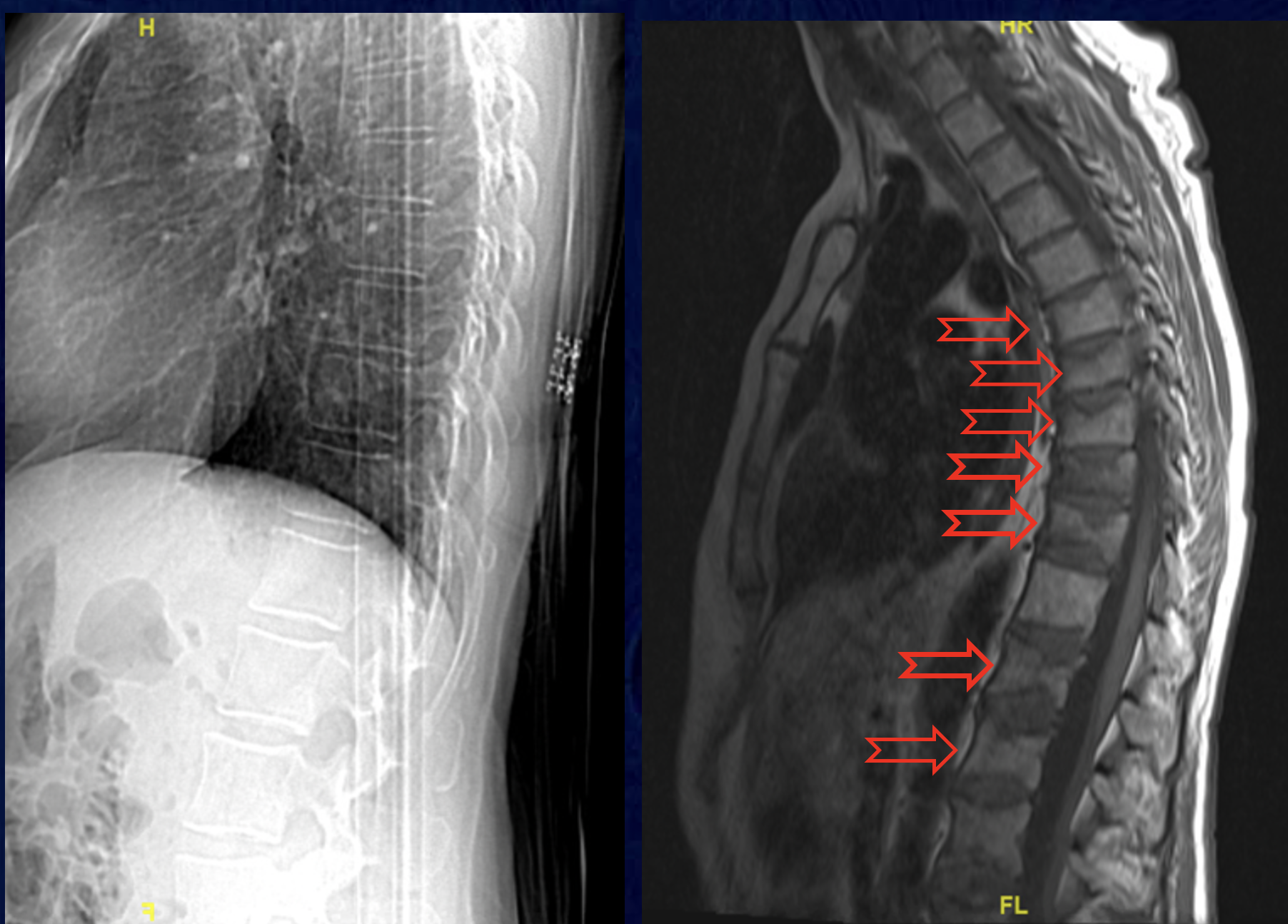 This pair of xrays is from a patient who skipped a dose of denosumab. Several months later she starting having bone pain, noticed height loss and kyphosis. A study of patients who had been on denosumab for longer than 3 years found 7% of the patients had multiple vertebral compression fractures, and almost half of them had 4 or more fractures. Two early papers describe severe multiple vertebral fractures after discontinuation (Aubry-Rozier, 2016, Popp, 2016).
This pair of xrays is from a patient who skipped a dose of denosumab. Several months later she starting having bone pain, noticed height loss and kyphosis. A study of patients who had been on denosumab for longer than 3 years found 7% of the patients had multiple vertebral compression fractures, and almost half of them had 4 or more fractures. Two early papers describe severe multiple vertebral fractures after discontinuation (Aubry-Rozier, 2016, Popp, 2016).
Atypical fractures The bone formation rate on bone biopsies after 2 or 3 years of treatment was zero (no tetracycline labels) in 36% of the patients and almost zero the in the rest (Reid I). Tetracycline was present in the urine so it is certain that the patients took and absorbed the tetracycline. It is not known (and widely debated) whether low bone formation would eventually make the bone more brittle; within the first 3 years this was not observed. In the extension of the FREEDOM trial there were 2 cases of atypical femur fractures after 5 years of denosumab (Papapoulos, 2015). Since the drug has been on the market there have been other reports of atypical fractures (Thompson, 2014, Villiers, 2013, Paparodis, 2013). The incidence of this complication in long-term users is still undetermined, because denosumab is a newer medicine than the bisphosphonates and has not been used by as many people.
Osteonecrosis of the jaw was seen in 2% of patients with metastatic cancer who were treated with high doses (compared to 1.4% in the zoledronic acid group). In the FREEDOM trial in women with osteoporosis there were 8 cases seen in the extension (Papapoulos, 2015).
The following .pdf documents are from the FDA webpage. The FDA approved a medication guide that should be read by all patients prior to receiving the drug.

The Prolia Medication Guide for patients
Package insert
Background for the FDA advisory committee
The optimal duration of denosumab use is not known. Clinical trials lasted 3 to 4 years with extensions up to ten years, and during that time the drug was safe and effective. However, the bone formation is markedly suppressed and this could lead to brittle bone after prolonged use.
An injection of denosumab lasts for 6 months. After that, if another dose is not given, the osteoclasts may rebound and resorb bone at a rapid rate. In one small study involving 31 subjects from one of the early trials, the bone density at the total hip increased by 4.4% over two years on denosumab, then decreased by 5.3% the year after denosumab was discontinued. It was restarted again and the bone density increased by about 5% during the next year (Miller, 2008). In a larger study of 128 women the hip bone density increased by 3.6% during 2 years on denosumab, and decreased to baseline after a year, and after that the bone loss was similar to a placebo group. The markers of bone resorption were higher after discontinuation than they were prior to treatment. Fractures were seen in 3% of women in the two years after discontinuation, which was the same rate as in placebo patients (Bone, 2011)
More recently, serious hypercalcemia has been reported in a patient who discontinued denosumab after ten years of use (Koldkjaer Solling, 2016).
It is not clear how often this happens or if anything should be done. One author suggested that treatment with a bisphosphonate or SERM might prevent the rebound in resorption, but there are not yet any studies.
This is a graph showing the bone density during the largest clinical trial (the FREEDOM study) along with an extension up to 8 years. The blue line shows patients taking denosumab; the grey line shows patients taking placebo, and the dashed line shows patients switched from placebo to denosumab. 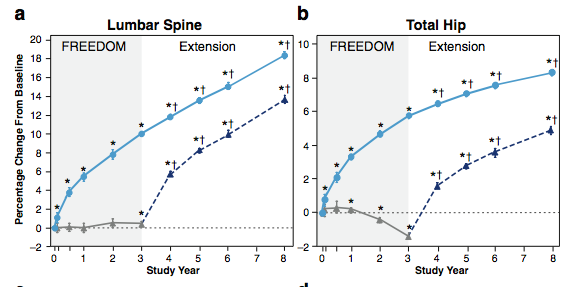
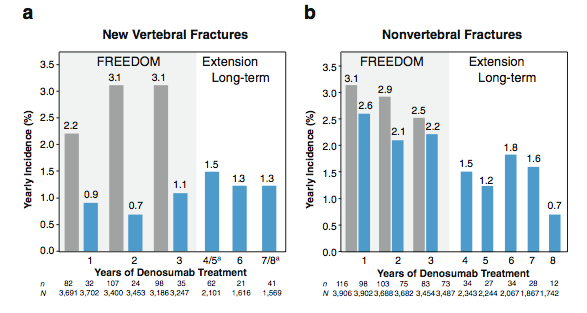
The bar graph shows the fracture rates in the treated (blue bars) vs. placebo group (grey bars) during the randomized part of the study, and then the treated patients during the extension. The fracture rates remained low.
Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporosis Int 2015;26(12):2773-83. link
A randomized clinical trial in patients with active rheumatoid arthritis who were receiving methotrexate included 226 patients. They were allowed to take bisphosphonates or glucocorticoids. All received supplemental calcium and vitamin D, depending on the initial levels. The change in erosion score measured by MRI was significantly better in the denosumab-treated patients compared to the placebo-treated patients (Cohen).
A sub-study of those who were receiving either bisphosphonates or glucocorticoids (N=75) found that the bone density increased in denosumab compared with placebo (Dore). The bone density after one year was similar in denosumab treated patients with or without glucocortoicoids. The combination of denosumab and alendronate was similar to denosumab alone and better than bisphosphonates alone.
The bone lesions in metastatic cancer follow a viscous cycle. The malignant cells secrete factors that stimulate the bone resorption. The bone contains growth factors that are released by resorption, and these growth factors further stimulate the malignant cells. The cancer cells can secrete RANK-ligand directly or indirectly by other factors that activate the osteoblasts. Thus, a medicine than blocks RANK-ligand theoretically should help to prevent the growth of these metastatic lesions.
Body and colleagues reported the results of a study comparing denosumab with zoledronic acid for the treatment of breast cancer patients with bone metastases. They randomized 2046 women and followed them for 34 months. The doses were higher than used in osteoporosis (denosumab 120mg or zoledronic acid 4mg every 4 weeks). The number of skeletal-related events was lower in the denosumab group and the time to the first event was delayed. The side effects related to infections were similar. The renal toxicity was 4.9%, compared to 8.5% in the zoledronic acid group.
Osteonecrosis of the jaw occurred in 20 denosumab-treated patients (2%) and 14 zoledronic acid-treated patients (1.4%).
Denosumab has also been studied in men with prostate cancer, patients with lung cancer, and patients with giant-cell tumor of the bone. It also increases bone density in women who are receiving aromatase inhibitors for non-metastatic breast cancer. In all of these reports the results were favorable.
In patients with multiple myeloma, randomized to denosumab or zoledronic acid, there was no difference in the time to a skeletal event (although with other cancers the time was longer in the denosumab group),. However, the relative hazard for mortality was 2.26 (1.13 to 4.50) (Henry, 2011). A newer study, however, showed benefit and the mortality was not increased.
Denosumab is not metabolized by the kidneys (unlike bisphosphonates) and that has led some people to say it can be used in these patients. Subsets of the patients in the FREEDOM study did have mild to moderately low eGFR and they showed benefit with this medicine. However, to be enrolled in that study the subjects had to have normal lab values, which are not the case in patients who have more serious kidney disease. In patients with eGFR lower than 30 the risk of severe hypocalcemia is increased. Calcium can decrease to levels as low as 5.3 mg/dl (1.34 nnol/L) (McCormick, 2012). In one medical center, after denosumab 6/8 patients with CKD-5 and 2/5 with CKD-4 developed severe hypocalcemia, with symptoms that included seizures, prolonged QT interval (Dave, 2015). In another case, a patient with myeloma and CKD was treated with denosumab and developed severe hypocalcemia, and during correction with calcitriol she rapidly developed soft tissue and vascular calcifications (Ueki, 2015).
 Click for more details about denosumab, bone histology, and physiology.
Click for more details about denosumab, bone histology, and physiology.

