Sclerostin (SOST)
Sclerosteosis
Van Buchen's Disease
SOST and wnt
Osteocytes and mechanotransduction
Animal studies that decrease sclerostin
Human studies of sclerostin antibodies
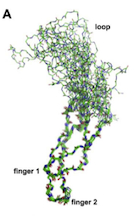
The SOST gene is located at 17q12-21, and codes for the protein sclerostin. Sclerostin is made primarily by osteocytes, and it inhibits bone formation and enhances apoptosis of osteoblasts. Patients with mutations in the SOST gene have very high bone density. SOST binds to LRP5 and inhibits the Wnt-signalling pathway. It is also a mild BMP antagonist.
Weidauer. Biochemical and Biophysical Res Comm. 2009;380:160
Sclerosteosis
Sclerosteosis is a serious, autosomal recessive disease with thick bones, entrapment of cranial nerves leading to deafness and facial nerve palsy, increased intracranial pressure, and frequently syndactyly. Patients are tall and heavy but not obese. Most patients are from South Africa and are homozygotic for a specific mutation in the SOST gene, which is mapped to 17q12-21.
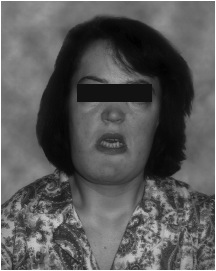
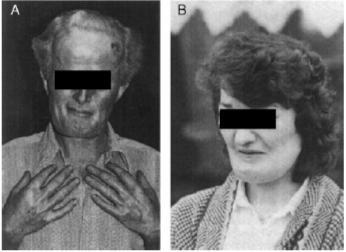
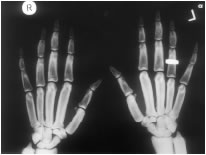 This figure from the review by Hamersma shows a woman with facial nerve palsy and thick, square mandible. The hand xrays demonstrate the thickened bones of the hands. from Hamersma H et al, 2003, The natural history of sclerosteosis. Clin Genet 63:192-7
used with permission.
This figure from the review by Hamersma shows a woman with facial nerve palsy and thick, square mandible. The hand xrays demonstrate the thickened bones of the hands. from Hamersma H et al, 2003, The natural history of sclerosteosis. Clin Genet 63:192-7
used with permission.
The authors note that none of their 63 patients have ever had a fractured bone. Also, the obligatory heterozygous gene carriers are resistant to fractures. The patients do not have degenerative osteoarthropathy.
Another report of two siblings who had several features of sclerosteosis showed a different mutation in the SOST gene. They also had thick bones, deafness, facial palsy, but not syndactyly.

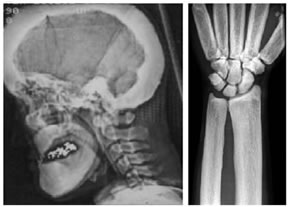
from Balemans W, et al. 2005. A generalized skeletal hyperostosis in two siblings caused by a novel mutation in the SOST gene. Bone 36:943-7. Used with permission
Van Buchem's Disease
This disease is very similar to sclerosteosis, but without the hand deformities. The mutations are located in a region downstream from the SOST gene. An autosomal dominant disease which is similar is caused by mutation in LRP-5. Beighton P, Barnard A, Hamersma H, van der Wouden A. The syndromic status of sclerosteosis and van Buchem disease. Clin Genet. 1984;25(2):175-81.. Used with permission
SOST and Wnt
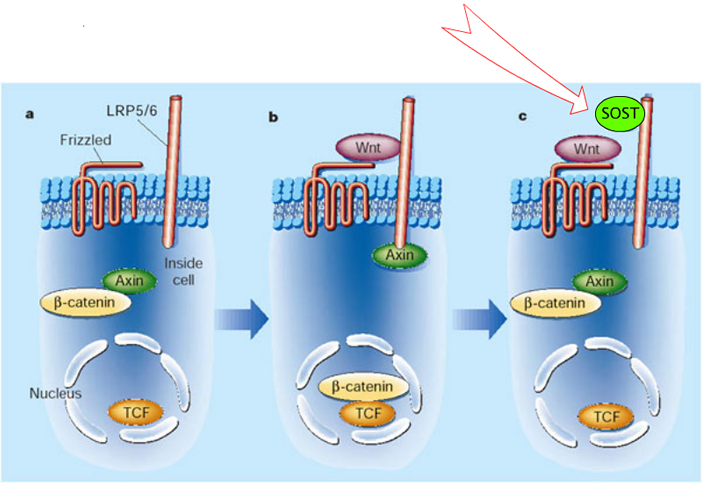
SOST is a homolog of WISE, which binds to LRP-6. SOST binds to LRP-5 which is a co-receptor in the Wnt-signalling pathway. Thus, SOST inhibits Wnt-signalling, similar to Dkk inhibition.
SOST in osteocytes and mechanotransduction
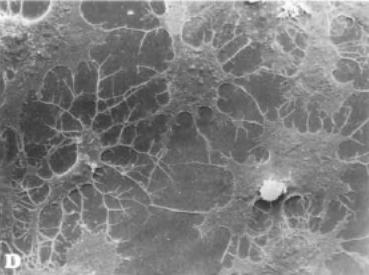 |
SOST is expressed mainly in osteocytes. These cells form a network which senses mechanical strain. Osteocytes can alter the secretion of sclerostin to regulate bone formation.
Osteocytes in culture showing extensive network of flattened cells. from: A van der Plas and PJ ijweide, Isolation and Purification of osteocytes. J Bone Mineral Res 1992;7:389-396. With permission from the American Society for Bone and Mineral Research. |
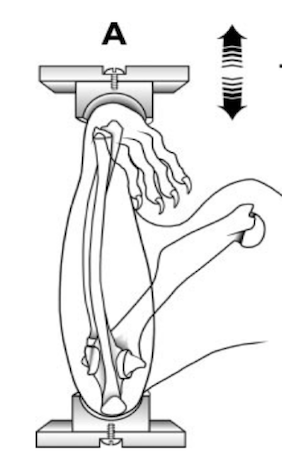
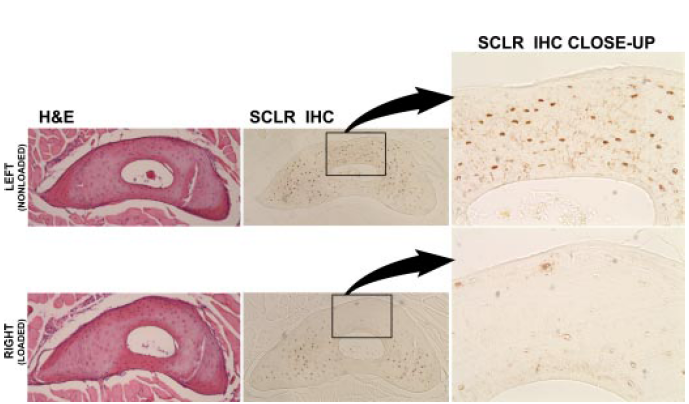
This diagram shows the design of an experiment in which the mouse ulna was either loaded or unloaded to see what happened to the osteocytes in the bone.
These sections show the ulna in the control mice (top) and the same area in the mice whose were loaded (lower). The H&E stains show that the osteocytes are viable. The close-up shows sclerostin staining, which is almost absent in the loaded arm.
Robling AG, Niziolek PJ, Baldridge et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/Sclerostin. J Biol Chem 2008;283:5866. Used with permission.
Animal studies that decrease sclerostin
|

|
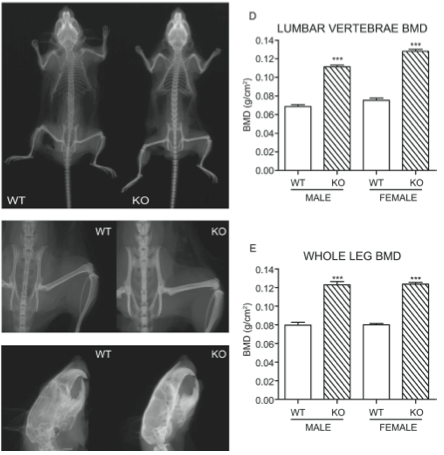 |
Mice with SOST knock-out had lower sclerostin levels and higher bone density as shown in these xrays.
Li X. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Min Res 2008:23:860 |
Treating rats with antibodies against sclerostin results in increased bone, shown in the xrays below, and the quality of the bone is good, with a lamellar pattern and clear tetracycline labels.
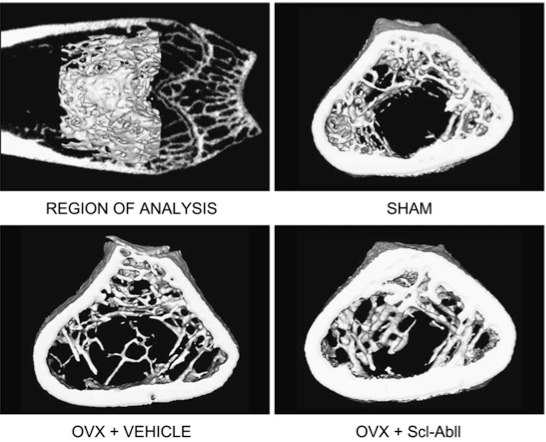
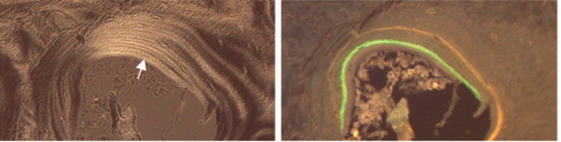
Li X Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Min Res 2009;24:578
Human studies of antibodies against sclerostin
There are two anti-sclerostin antibodies that have been studied in humans. Here's what their names mean:
ROM, BL = unique prefix assigned to the new drug
OS = works on the bone
OZU = humanized
MAB = monoclonal antibody
So we have ROMOSOZUMAB and BLOSOZUMAB
The first pilot study was reported by Padhi. They treated 72 healthy subjects with a single injection of a placebo or the antibody, and saw dose-related increases in the bone density, increases in the bone formation rates, and decreases in the bone resorption rates.
Similar benefits from initial studies of blosozumab were reported by McColm.
A new study was done in 419 postmenopausal women with low bone density. They were randomized to placebo, various doses of romosozumab, or alendronate or teriparatide for a year. The bone density at the spine increased by 11.3% with the highest dose that they studied. The markers of bone formation increased transiently and the markers of bone resorption were sustained for the year. No serious side effects were reported.
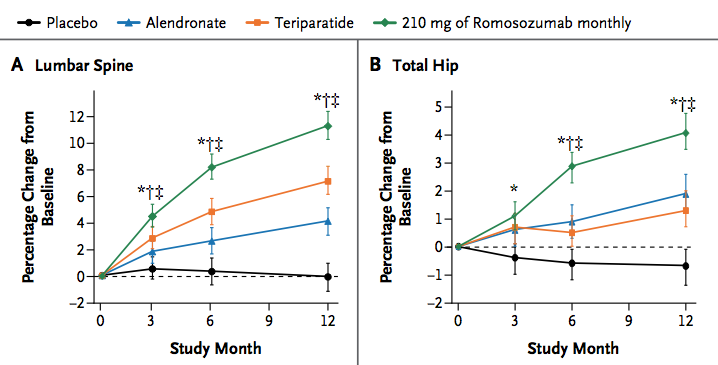
McClung MR Romosozumab in Postmenopausal Women with Low Bone Mineral Density. New Engl J Med 2014;370;412. Used with permission.
 Feb 2016 there was a press release that a phase 3 study, lasting for a year, showed fracture reduction. There weren't many details in this press release, but it sounded like side effects were minimal.
Feb 2016 there was a press release that a phase 3 study, lasting for a year, showed fracture reduction. There weren't many details in this press release, but it sounded like side effects were minimal.
Updated 3/5/16



 This figure from the review by Hamersma shows a woman with facial nerve palsy and thick, square mandible. The hand xrays demonstrate the thickened bones of the hands. from Hamersma H et al, 2003, The natural history of sclerosteosis. Clin Genet 63:192-7
used with permission.
This figure from the review by Hamersma shows a woman with facial nerve palsy and thick, square mandible. The hand xrays demonstrate the thickened bones of the hands. from Hamersma H et al, 2003, The natural history of sclerosteosis. Clin Genet 63:192-7
used with permission.













 Feb 2016 there was a press release that a phase 3 study, lasting for a year, showed fracture reduction. There weren't many details in this press release, but it sounded like side effects were minimal.
Feb 2016 there was a press release that a phase 3 study, lasting for a year, showed fracture reduction. There weren't many details in this press release, but it sounded like side effects were minimal. 
