Regulation of Water Balance
Water Reabsorption
Water reabsorption is a passive process: water is reabsorbed by osmosis. In most of the nephron
there is unregulated isosmotic reabsorption of water and solute,
in other words, water reabsorption is coupled to solute
reabsorption. However, it is possible to reabsorb water
independently of solute to produce a concentrated
urine, that is, urine that has a
higher osmolarity than the extracellular fluid.
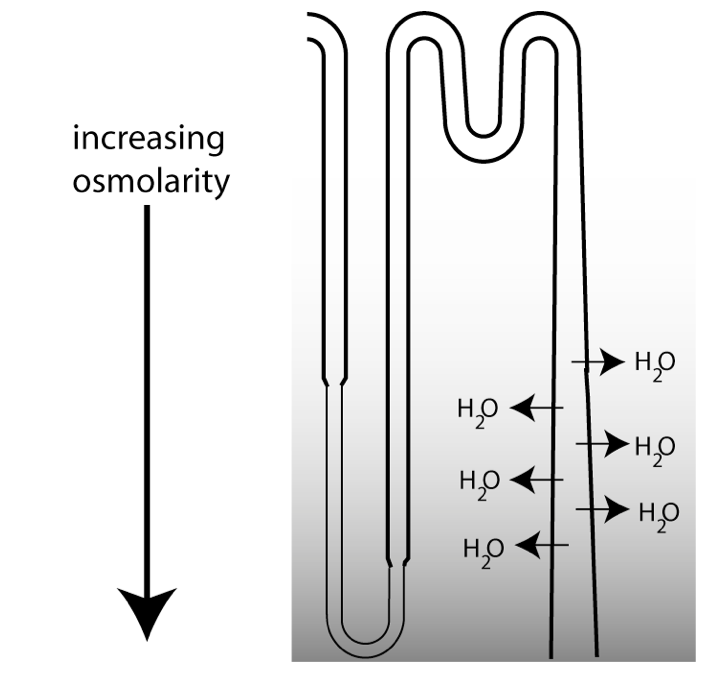 Regulated
water reabsorption occurs from the medullary
collecting duct.
Regulated
water reabsorption occurs from the medullary
collecting duct.
The figure at left is a schematic showing the last part of a
nephron. The ability to excrete urine that is more
concentrated than the extracellular fluid (ECF) depends upon the loops of Henle, which function to
concentrate osmolarity in the deepest part of the medulla,
creating a vertical osmotic gradient. As the collecting duct
descends through the medulla, the increasing osmolarity in the
surrounding interstitial fluid drives water reabsorption.
However, there is a tremendous variability in water excretion.
The urine produced can either be concentrated, or very dilute. How
do the kidneys vary their urine concentrating ability? They do so
by regulating water permeability in the collecting duct.
Regulated Permeability in the Collecting Duct
In humans, the vertical osmotic gradient in the medulla allows
the kidneys to produce urine that can be roughly 5 times as
concentrated as the ECF. Urine concentration can be varied through
the regulation of water permeability in the collecting duct.
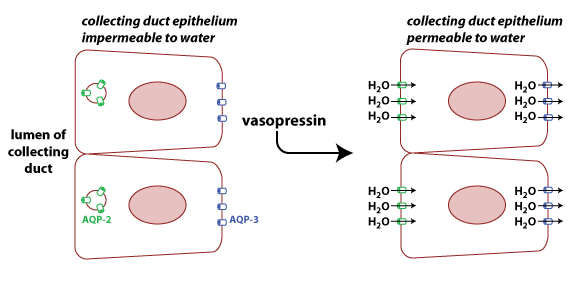 The permeability
of cell membranes to water depends upon the presence of water
channels known as aquaporins.
There is a family of aquaporin proteins, with different types
being expressed in different tissues. AQP3 (blue in figure) is
constitutively expressed on the basolateral surface of cells in
the collecting duct. AQP2 is found on the apical surface of these
cells, but the number of AQP2 channels on the membrane is
regulated by the hormone vasopressin
(also known arginine vasopressin
and as antidiuretic hormone or ADH). When vasopressin
binds to its receptor on the collecting duct cells, it stimulates
the translocation of AQP2 to the membrane by causing vesicles
containing the protein to fuse with the plasma membrane. The
result is more AQP2 proteins on the apical membrane and higher
permeability to water.
The permeability
of cell membranes to water depends upon the presence of water
channels known as aquaporins.
There is a family of aquaporin proteins, with different types
being expressed in different tissues. AQP3 (blue in figure) is
constitutively expressed on the basolateral surface of cells in
the collecting duct. AQP2 is found on the apical surface of these
cells, but the number of AQP2 channels on the membrane is
regulated by the hormone vasopressin
(also known arginine vasopressin
and as antidiuretic hormone or ADH). When vasopressin
binds to its receptor on the collecting duct cells, it stimulates
the translocation of AQP2 to the membrane by causing vesicles
containing the protein to fuse with the plasma membrane. The
result is more AQP2 proteins on the apical membrane and higher
permeability to water.
Regulation of Vasopressin Secretion
Vasopressin is a peptide hormone that is produced by neurosecretory cells, a type of
endocrine cell found in the hypothalamus.
As
shown in the figure, neurosecretory cells have dendrites, axons,
and terminals just like typical neurons. The difference is that
the terminals of neurosecretory cells are adjacent to capillaries.
Neurosecretory cells secrete regulatory molecules (green dots)
that enter the circulation and act as hormones.
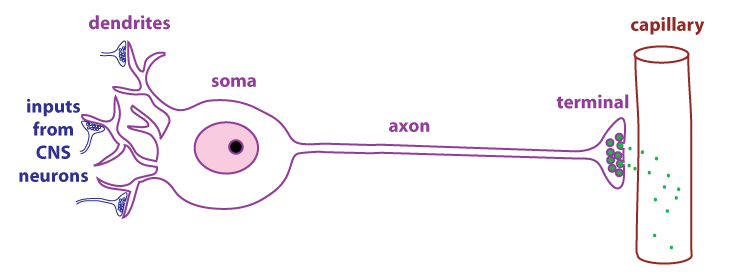
Vasopressin is secreted by neurosecretory cells whose cell bodies
are in the hypothalamus and whose terminals are located in the posterior pituitary (also called
the neurohypophysis). The main control of vasopressin secretion is
by the osmoreceptors, neurons
that sense changes in the osmolarity of the extracellular fluid.
The osmoreceptors are also located in the hypothalamus. If
the osmolarity of the ECF increases, the osmoreceptors increase
their frequency of action potential firing, and more vasopressin
is secreted. Increased action potential firing by the
osmoreceptors also stimulates thirst.
If
the osmolarity of the ECF decreases, the osmoreceptors decrease
their action potential frequency and less vasopressin is secreted.
Disorders in the Ability to Concentrate Urine
(AVP-D and AVP-R)
If there is a problem with vasopressin action, the result is an
inability to concentrate urine, which leads to polyuria (a high urine
volume). This can be caused by a lack of vasopressin (arginine vasopressin deficiency* or
AVP-D; formerly known as central
diabetes insipidus) or due to a
defect in the ability of the kidney to respond to vasopressin (arginine vasopressin resistance or AVP-R;
formerly known as nephrogenic
diabetes insipidus). AVP-D may be
caused by a genetic mutation where vasopressin is missing or
defective. Head trauma, a tumor, or injury to the posterior
pituitary may also cause AVP-D. AVP-D is treated with
desmopressin, a synthetic vasopressin agonist.
AVP-R may be caused by a defect in the vasopressin receptor.
Another type of mutation that causes the disorder involves a
defect in the gene for AQP2. This defect prevents the proper
localization of AQP2 proteins on the apical membrane of collecting
duct cells. The drug lithium, which is used in the treatment of
bipolar disorder, can cause acquired AVP-R.
*Clinicians refer to vasopressin as arginine vasopressin, the
human form of vasopressin which contains an arginine in the 8th
position of the nine-amino acid vasopressin peptide.
Summary: Homeostasis of ECF Osmolarity
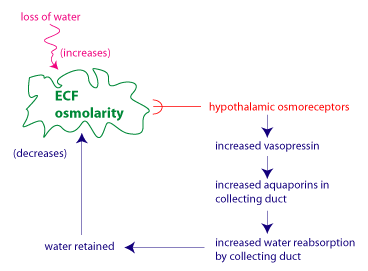
The figure illustrates the regulation of water balance as a
negative feedback regulatory system. The regulated variable is the
ECF osmolarity. The sensors are the hypothalamic osmoreceptors,
which modulate their frequency of action potential firing in
response to changes in ECF osmolarity. The effector system that
restores ECF osmolarity to its set point involves vasopressin and
its effects on water reabsorption in the collecting duct.
Optional
A group of endocrinologists from all over the world are pushing
to change the name "diabetes insipidus" to more accurately
reflect the etiology of the disorder, and mostly to prevent
confusion with diabetes mellitus, which can lead to disastrous
consequences for patients. Here is a link to the
publication describing the rationale for changing the name of
central diabetes insipidus to arginine vasopressin deficiency
(AVP-D) and nephrogenic diabetes insipidus to arginine
vasopressin resistance (AVP-R).
Arima, H. et al. (2022) "Changing the Name of Diabetes
Insipidus: A Position Statement of the Working Group for
Renaming Diabetes Insipidus " the Journal of Clinical
Endocrinology and Metabolism 108: 1-3 link
to article
 Regulated
water reabsorption occurs from the medullary
collecting duct.
Regulated
water reabsorption occurs from the medullary
collecting duct. The permeability
of cell membranes to water depends upon the presence of water
channels known as aquaporins.
There is a family of aquaporin proteins, with different types
being expressed in different tissues. AQP3 (blue in figure) is
constitutively expressed on the basolateral surface of cells in
the collecting duct. AQP2 is found on the apical surface of these
cells, but the number of AQP2 channels on the membrane is
regulated by the hormone vasopressin
(also known arginine vasopressin
and as antidiuretic hormone or ADH). When vasopressin
binds to its receptor on the collecting duct cells, it stimulates
the translocation of AQP2 to the membrane by causing vesicles
containing the protein to fuse with the plasma membrane. The
result is more AQP2 proteins on the apical membrane and higher
permeability to water.
The permeability
of cell membranes to water depends upon the presence of water
channels known as aquaporins.
There is a family of aquaporin proteins, with different types
being expressed in different tissues. AQP3 (blue in figure) is
constitutively expressed on the basolateral surface of cells in
the collecting duct. AQP2 is found on the apical surface of these
cells, but the number of AQP2 channels on the membrane is
regulated by the hormone vasopressin
(also known arginine vasopressin
and as antidiuretic hormone or ADH). When vasopressin
binds to its receptor on the collecting duct cells, it stimulates
the translocation of AQP2 to the membrane by causing vesicles
containing the protein to fuse with the plasma membrane. The
result is more AQP2 proteins on the apical membrane and higher
permeability to water.
