Body weight is a function of the balance between energy
intake (kilocalories in fat, carbohydrate and protein from
our diet) and energy expenditure (kilocaries burned in
cellular maintenance and muscle contraction). Energy homeostasis refers to
homeostatic control of the balance between energy intake and
energy expenditure. Energy homeostasis involves complex
mechanisms that control hunger, satiety, and metabolic rate.
The hormone leptin is an important component in the regulation of energy homeostasis. Leptin participates in a negative feedback loop that regulates the amount of energy stored in adipose tissue (adiposity), which is a major determinant of body weight. Leptin is secreted by adipocytes (fat cells). Leptin secretion increases when the amount of adipose tissue increases, signaling that there is increased energy stored in the body.
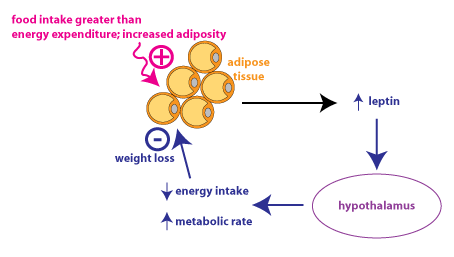
Leptin is transported into the brain, primarily acting on neurons in the hypothalamus. Increased leptin causes decreased energy intake (feeding). Leptin is not thought to be a satiety signal, which would be a factor that would cause a person to end a meal because he feels “full”. Instead, leptin is thought to act over a longer time course to enhance the response to satiety signals. Leptin also affects energy expenditure through various mechanisms. Increased leptin also causes an increase in metabolic rate.
Leptin was discovered through studies of the obese mutant mouse. The obese mutation is caused by a deletion of the gene encoding leptin, so homozygous mutant mice completely lack leptin. These mutant mice eat constantly, are incredibly obese, and develop diabetes mellitus due to insulin resistance. They are also infertile, indicating that leptin is a signal that is required for activation of the reproductive axis. Injections of leptin very effectively causes weight loss and improved insulin sensitivity in obese mutant mice.
There was much excitement after the discovery of leptin because it was hoped that it might be an effective weight loss therapy in humans. Unfortunately, human obesity is not caused by leptin deficiency. In fact, obese people generally have higher levels of circulating leptin, reflecting their greater amount of adipose tissue. In clinical trials, leptin was not able to cause weight loss except at very high doses. One hypothesis is that leptin resistance--meaning a reduced response to leptin-occurs in obesity.
Another way of understanding the disappointing results for leptin as a weight loss therapy is to consider that during evolution, this negative feedback loop was probably more important for preventing starvation rather than overfeeding. Scarcity of food was a significant factor during human evolution, whereas food overabundance is only a recent phenomenon. Thus, our physiology may be more sensitive to decreases in leptin signaling.
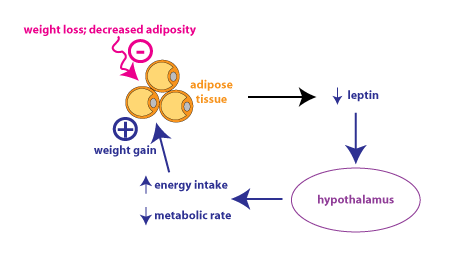
The above figure shows the leptin feedback loop in response to
weight loss, as might occur in someone who is dieting. This shows
why it is so difficult for dieters to keep weight off: decreased
leptin secretion leads to increased hunger and decreased metabolic
rate. Leptin might be effective therapeutically if it was
administered after weight loss, where it could counteract the
decrease in leptin secretion and prevent the changes that make it
so hard to maintain weight loss. At present, leptin is not
an approved weight-loss therapy.
GLP-1 is a peptide hormone that is released by endocrine cells located in the epithelium of the small intestine. GLP-1 is an incretin hormone, meaning it acts on cells in the pancreas to increase glucose-dependent insulin secretion. GLP-1 agonist drugs have been used to treat type 2 diabetes mellitus since 2005. It was noted that patients using GLP-1 agonists tended to lose weight. Furthermore, GLP-1 levels increase following bariatric surgery and it is thought that these increased GLP-1 levels play some role in the changes in appetite that occur following bariatric surgery. There are two GLP-1 agonists that have been approved for weight loss treatment: liraglutide (Saxenda™) and semaglutide (Wegovy™). A third drug, Tirzepatide (Mounjaro) is a dual GIP/GLP-1 agonist that has been approved for treatment of type 2 diabetes and is being investigated as a weight loss therapy. These drugs are peptides, so they need to be administered by injection.
The New England Journal of Medicine series "Double Takes" is a
new video series that explores medical topics, providing patient
perspectives as well as scientific and clinical
background. There is an excellent short video series about
weight and health.
This link takes you to the series about weight and health: Weight and Health—Pathophysiologies and Therapies(total run time 13:16)
This link takes you to the landing page for Double Takes:
Double Takes Video Series