Polycystic ovary syndrome
Description
Polycystic ovary syndrome (PCOS) is the most common endocrine
disorder among women of reproductive age, with an estimated
prevalence of 6-10%. PCOS is the most common cause of anovulatory infertility (meaning infertility due to a lack
of ovulation). PCOS is classified as a syndrome because it is a heterogeneous
disorder: not all women with PCOS express all the features
associated with the disorder.
PCOS is diagnosed when a woman has 2 out of 3 diagnostic
characteristics:
- hyperandrogenism
Hyperandrogenism is excess secretion of androgens. This
can be measured biochemically, but also may cause clinical
symptoms. Hyperandrogenism may cause hirsutism, which is a masculine
pattern of hair growth on the body, and acne,
because androgens have an effect on the sebaceous glands of the
skin that promotes acne. Androgens may also cause hair loss on
the scalp (alopecia).
- problems with ovulation
Problems with ovulation affect the timing of menstruation.
A woman might experience oligomenorrhea
(irregular menstruation where cycles are longer than 35 days) or
amenorrhea (a lack of
menstruation).
- polycystic ovarian morphology
PCOS gets its name from changes in the ovary. In
polycystic ovaries, the ovaries are enlarged and contain
multiple immature follicles (greater than 24 per ovary).
Diagnosis also involves tests that exclude other causes of
hyperandrogenism and anovulation.
There are also metabolic disturbances associated with PCOS.
Frequently, women with PCOS are found to be insulin
resistant. Because insulin resistance is a
decreased sensitivity to insulin, this means that more insulin is
necessary to achieve the same effect. For this reason, individuals
who are insulin resistant have higher levels of insulin secretion
or hyperinsulinemia. Because
women with PCOS are insulin resistant, they are at a greater risk
for developing type 2 diabetes mellitus
(T2DM). Many women with PCOS may be overweight, which can
contribute to their insulin resistance and risk for T2DM.
Endocrine disturbances in PCOS
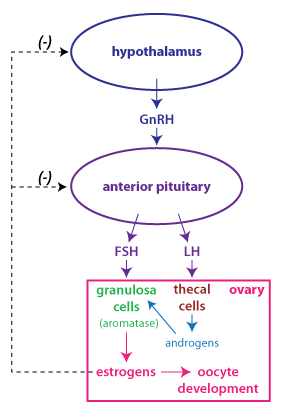 Normal follicle
development begins when estrogen and progesterone levels drop due
to degeneration of the corpus luteum. The release from negative
feedback inhibition allows a small but steady increase in FSH and
LH levels that stimulates the growth phase for a group of
follicles. In the early follicular phase, granulosa
cells respond to FSH only, while thecal cells respond to LH. The
hormonal interactions in the early follicular phase are shown in
the figure at right.
Normal follicle
development begins when estrogen and progesterone levels drop due
to degeneration of the corpus luteum. The release from negative
feedback inhibition allows a small but steady increase in FSH and
LH levels that stimulates the growth phase for a group of
follicles. In the early follicular phase, granulosa
cells respond to FSH only, while thecal cells respond to LH. The
hormonal interactions in the early follicular phase are shown in
the figure at right.
The cause of PCOS is not at all clear, but one consistent
observation is that there is an imbalance in gonadotropin
production. LH secretion is elevated,
while FSH secretion is the same, or even decreased. LH stimulates theca cell proliferation and
secretion of androgens, but there is insufficient FSH to
stimulate granulosa cells. Recall that
production of estrogen by
the ovary requires the activity of the enzyme aromatase that is expressed in
granulosa cells. The result is high levels of androgens secreted
from the ovary (hyperandrogenism), and a failure of follicle
development to progress.
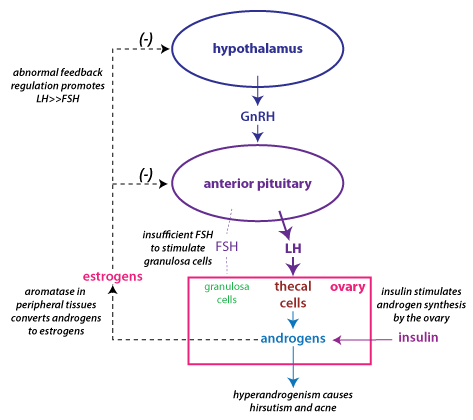
The figure above depicts how the endocrine disturbances in PCOS
become part of a vicious cycle, where the abnormalities are
reinforced. The androgens secreted from the ovary are converted to
estrogen because certain body tissues (in particular, adipose
tissue) express aromatase. This continuous level of estrogen
causes abnormal feedback regulation of gonadotropin
secretion, such that LH secretion continues to be high relative to
FSH secretion. Hyperinsulinemia contributes to the problem because
insulin stimulates ovarian androgen production.
Treatment for PCOS in women who don't want to get
pregnant
Hormonal contraceptives
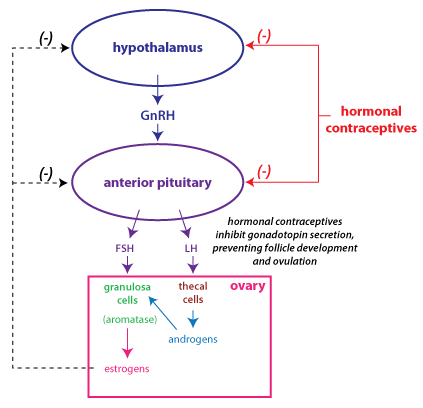 If a woman is not
seeking to get pregnant, PCOS is often treated with hormonal contraceptives. The
goal in treatment is to decrease hyperandrogenism and to address
the negative effects of PCOS on the endometrium of the uterus.
If a woman is not
seeking to get pregnant, PCOS is often treated with hormonal contraceptives. The
goal in treatment is to decrease hyperandrogenism and to address
the negative effects of PCOS on the endometrium of the uterus.
Typically, hormonal contraceptives contain a low dose of
estrogen and progesterone, and are taken for 3 weeks, with one
week off for a "withdrawal bleed". The estrogen and progesterone
act to restore normal negative feedback regulation and lower LH
secretion. This is often sufficient to reduce hyperandrogenism
and its associated symptoms.
Hormonal contraceptives are also useful for preventing uterine
problems. In untreated PCOS, the endometrium experiences unopposed estrogen: that
is, high levels of estrogen with no progesterone, because the
cycle doesn't advance to the luteal phase. Recall that estrogen
stimulates endometrial proliferation, while high levels of
progesterone (as occur during the luteal phase) will stop
proliferation and promote endometrial secretion. Long
stretches of unopposed estrogen will promote too much
endometrial proliferation, and can cause a woman to have
excessive menstrual bleeding when she does have a period.
Increased stimulation of endometrial proliferation (as occurs in
PCOS) also increases a woman's risk for the development of endometrial
cancer.
Treatment for PCOS in women who want to become
pregnant (ovulation induction)
Letrozole
If a woman is
seeking to become pregnant, the first line of therapy is
treatment with letrozole.
Letrozole is an aromatase
inhibitor that is approved for the treatment of
breast cancer in post-menopausal women. Letrozole is not
FDA approved for ovulation induction, but it has been found to
be an effective treatment, and is now the first line of
treatment because of its superiority to clomiphene in rates of
pregnancy and live births. Letrozole prevents the conversion of
androgens to estrogens. Letrozole works to induce ovulation by
limiting estrogenís negative feedback inhibition of gonadotropin
secretion.
Clomiphene
Clomiphene is an older
drug, which used to be the first line of treatment for ovulation
induction. Clomiphene acts as an estrogen
antagonist in the hypothalamus and anterior
pituitary. It prevents the negative feedback effect of
estrogen, thus allowing FSH secretion to increase so that
follicle development can be stimulated.
A potential advantage of letrozole over clomiphene is that it
has a shorter half-life, allowing normal estrogen action later
in the cycle. In the mid-follicular phase, negative feedback
from estrogen limits gonadotropin secretion so that only a
single follicle becomes dominant. Thus, ovulation induction with
aromatase inhibitors should have less risk than clomiphene for
inducing multiple ovulations. As well, the shorter half-life
allows more estrogen stimulation of endometrial development
during the proliferative phase in the uterus.
Metformin
This treatment approach addresses the problem of insulin
resistance. Metformin is a treatment for type 2 diabetes
mellitus that works to improve insulin sensitivity.
Studies show that in women who are insulin resistant, metformin
is apparently safe and effective in lowering insulin and
androgen levels, and may increase ovulation. However
metformin is not recommended as a first-line therapy for
ovulation induction, because it is much less effective than
letrozole or clompiphene.
Weight loss improves insulin sensitivity, and can also restore
normal ovulatory cycles in some women with PCOS.
FSH
In some women, clomiphene and other treatments are not
successful at inducing ovulation. In this case, exogenous FSH is
needed to stimulate follicle development. The first treatment
developed was menotropin, a mixture of gonadotropins
purified from the urine of menopausal women. (Can you think why
this would be a particularly rich source of FSH and LH?)
Although menotropin contains LH, it is really the FSH that is
important for stimulating ovulation in women with PCOS. Urofollitropin
is FSH purified from menopausal urine. More recently, purified
recombinant FSH (follitropin) has been produced.
Treatment with FSH is more expensive, and involves more risk.
One problem is that high levels of FSH may induce multiple
ovulations and cause higher order pregnancies (i.e. twins or
triplets) which are risky for the mother and the developing
fetuses. Another problem is ovarian
hyperstimulation syndrome, a dangerous condition
that arises when the ovary is stimulated so that multiple
follicles mature. During ovarian hyperstimulation syndrome there
is an increase in vascular permeability that leads to edema,
nausea, and abdominal pain. If severe, it can result in clotting
abnormalities, respiratory distress, and renal failure. Because
of the risk of ovarian hyperstimulation, a woman treated with
gonadotropins needs to be carefully monitored with transvaginal
ultrasound (to monitor the number of developing follicles) and
for excessive increases in estrogen secretion.
 Normal follicle
development begins when estrogen and progesterone levels drop due
to degeneration of the corpus luteum. The release from negative
feedback inhibition allows a small but steady increase in FSH and
LH levels that stimulates the growth phase for a group of
follicles. In the early follicular phase, granulosa
cells respond to FSH only, while thecal cells respond to LH. The
hormonal interactions in the early follicular phase are shown in
the figure at right.
Normal follicle
development begins when estrogen and progesterone levels drop due
to degeneration of the corpus luteum. The release from negative
feedback inhibition allows a small but steady increase in FSH and
LH levels that stimulates the growth phase for a group of
follicles. In the early follicular phase, granulosa
cells respond to FSH only, while thecal cells respond to LH. The
hormonal interactions in the early follicular phase are shown in
the figure at right.
 If a woman is not
seeking to get pregnant, PCOS is often treated with hormonal contraceptives. The
goal in treatment is to decrease hyperandrogenism and to address
the negative effects of PCOS on the endometrium of the uterus.
If a woman is not
seeking to get pregnant, PCOS is often treated with hormonal contraceptives. The
goal in treatment is to decrease hyperandrogenism and to address
the negative effects of PCOS on the endometrium of the uterus.