
Water Resources and Pollution:
Estuarine Field Studies
University of Washington, Tacoma
TESC 431: Spring 2006

 |
Water Resources and Pollution:Estuarine Field StudiesUniversity of Washington, TacomaTESC 431: Spring 2006 |
 |
| Main | Commencement Bay | Quartermaster Harbor | Hood Canal | San Juan Islands |
General Collection Methods
Conductivity-Temperature-Depth (CTD):
The CTD determines water properties and provides a depth profile of salinity by measuring conductivity of the seawater. A temperature profile is obtained with an electrical resistance thermometer, and depth readings are made with a pressure sensor. The CTD was lowered into the water in meter increments. It was enclosed in a protective cage that contained a series of either 2.5 L or 4.0 L Niskin bottles, which were closed by sending a messenger down the line in order to collect samples at varying depths in the water column. The CTD had other sensors such as fluorescence, dissolved oxygen and transmission sensors. These water properties were monitored continuously throughout the water column. The data was stored onboard the CTD instrument and downloaded once aboard the research vessel.
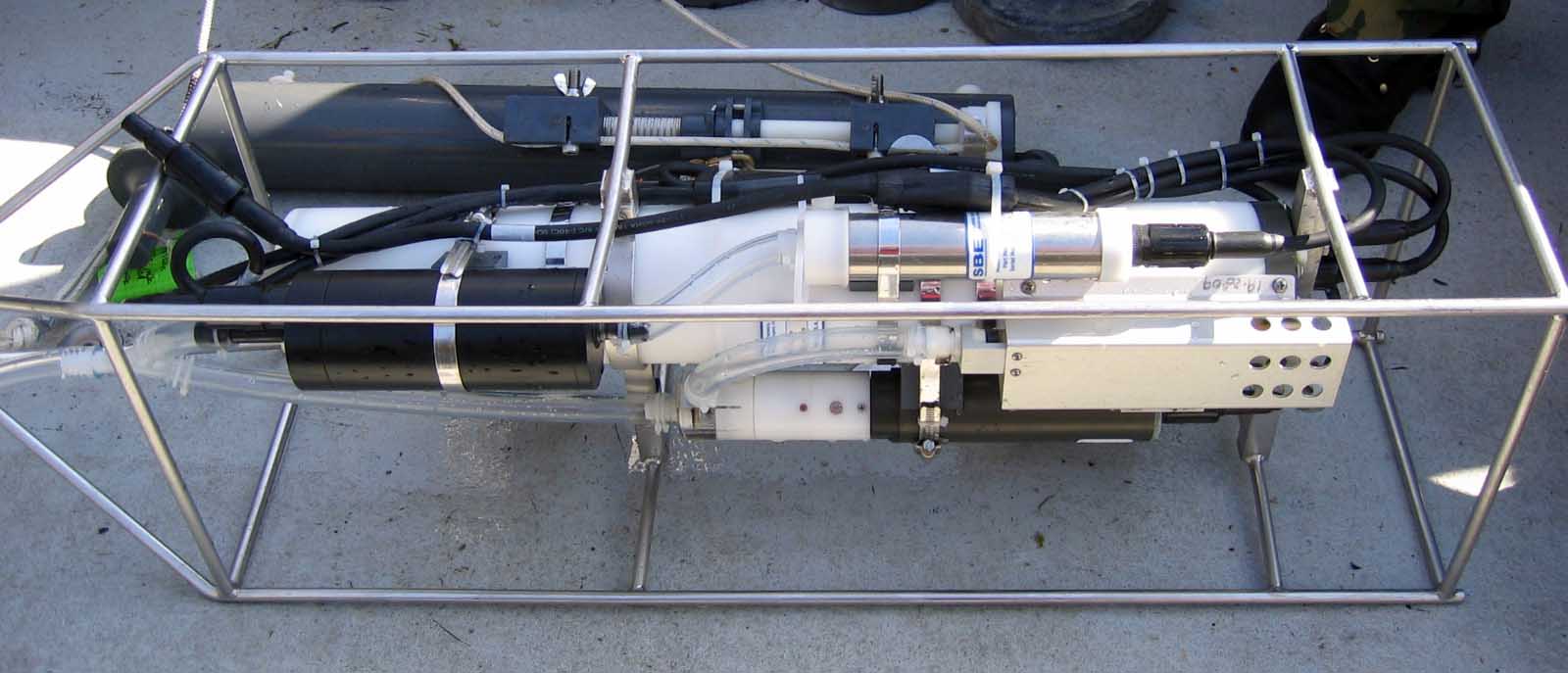

Niskin Bottle:
The Niskin bottle used was 4.0 or 2.5 liters and was either attached on the same line approximately one meter above the CTD or was lowered manually in meter increments to the desired depth. This instrument is utilized to collect individual water samples and analyze water chemistry. More specifically, the Niskin bottle was used to collect dissolved oxygen, chlorophyll and nutrient samples. Once the Niskin bottle and CTD were at the desired depth the messenger was sent down the line to trigger closing of the bottle lids. The following chemical properties were analyzed:


Chlorophyll a: Replicate samples were taken at each sampling station, two of which were collected at depth and two at the surface. The samples were stored on ice for transport. In the lab, samples were filtered through glass fiber filters with a vacuum pump. The filter paper collected the chlorophyll, which was subsequently placed in a 10 ml solution of acetone and sonicated to burst the cells. Once the cells burst, the chlorophyll was released into the acetone. After sonication, the samples remained stationary for ten minutes and were then centrifuged for five minutes. The water at the top was extracted and placed inside the fluorometer, which measured for chlorophyll in mg/m³ (Strickland and Parsons 1972).
Dissolved oxygen: Replicate samples were taken at each sampling station, two of which were collected at depth and two at the surface. The samples were collected in pre-measured flasks that were sealed with a stopper. Water was drawn from the Niskin bottle through a rubber hose to prevent exposure to atmospheric oxygen. Once filled, the flasks were injected with 1 ml of manganous chloride and 1 ml of alkaline sodium iodide reagents. The stopper was placed in the flask, its contents shaken and stored in the dark for transport to the laboratory. At the lab, the remaining steps of the Winkler Titration Method (Carpenter 1966) were performed using a Dosimat automatic titrator . Once a standard was established, the samples were titrated as follows: add stir bar to flask with collected sample, add 1 ml of sulfuric acid, place bottle on stirrer, titrate sample by adding thiosulfate, when the sample turns a straw yellow in color add 1 ml of starch and titrate to endpoint which is when the sample became clear.
Nutrients: One surface and one at depth sample was collected at each sampling station. Samples of approximately 30 ml were forced through a micro filter to remove suspended solids. The bottles were frozen and sent to the University of Washington Marine Chemistry Lab to be analyzed.
Secchi Disk:
The Secchi disk was 8-inches in diameter with alternating black and white quadrants. This instrument is used to measure light attenuation in surface water. The disk is lowered on a line in ½ meter increments to the depth at which it disappears from view. When the disk is no longer visible to the human eye, the depth was recorded. Measurements were taken at each of the sampling stations. All of the surface measurements were collected off the side of the research vessel with consistent light.
LICOR Meter:
The Licor Meter was a component of the CTD used by the Hood Canal Salmon Enhancement Group. Licor is the company that manufactures the light meter, a device used to measure light transmission. As sunlight radiates and passes through the water column, it is absorbed by primary producers, backscattered and turned into heat. Light transmission decreases with depth, and as a result there is less energy for photosynthetic organisms. Using the light meter, one can find the 99% absorbance level, where plankton can no longer survive off sunlight alone.
Plankton Samples:
A plankton tow net was used to collect dense plankton samples. Since volume of water towed was not known, the tow net samples did not determine plankton abundance, but rather provided a means of determining plankton species present and their relative abundance. The mesh on the plankton net was 20 microns.
A closing plankton net was used to collect deep samples of zooplankton. Weighted at the bottom, the net was lowed into the water to apredetermined depth. Once at the desired depth, the net was pulled up toward the surface and a messenger was sent down to close the net. By recording the distance that the net was net was pulled, and the volume of water towed can be calculated using the radius of the net's ring. This provides data for zooplankton density at certain depths.
The Niskin bottle was used to gather and examine types of plankton. Either a 2.5L or 4.0L Niskin bottle was filtered through a 20 micron net mesh. Each sample was collected at the surface. Plankton samples were counted using a compound microscope with ocular capacity. The phytoplankton samples were placed onto a Palmer Counting Slide, which holds 0.1 ml of fluid. The types of phytoplankton were counted and recorded.
Van Veen Grab Sampler and Grain Size Analysis Sieve:
The Van Veen Grab Sampler is a device used to collect samples of bottom sediment from the ocean floor. Once collected, the samples were stored on ice until further analysis could be completed. Sediment particle sizes were analyzed with a shaker sieve device. The sediment was stored in 60 degree conditions for 24 hours to remove moisture. Once the sample was dried, the weight was recorded. The sample was then placed in a six tiered system of varying size sieves in which it is shaken for a period of 30 minutes. The sediment falls through each layer of the sieve until it is too large too proceed. The weight is then recorded for each layer.



References
Carpenter, J.H. 1966. New measurements of oxygen solubility in pure and natural waters. Limnology and Oceanography 11: 264-277.
Strickland, J.D.H.; Parsons, T.R. 1972. A Practical Handbook of Seawater Analysis. The Alger Press Ltd. Ottowa
| Commencement Bay | Quartermaster Harbor | Hood Canal | San Juan Islands |
| Main |
Web page created by Alexander Abrahamson, Brandon Kemperman and Lisa Diment