- Indications
- Contraindications
- Dose, duration, and need for followup medicines
- Side effects
- Summary of clinical trials
- Combination with other drugs
- References
This page was written by Dr. Radhika Narla and edited by Dr. Ott

|
This page was written by Dr. Radhika Narla and edited by Dr. Ott |
Abaloparatide is related to parathyroid hormone (PTH), which is naturally an 84-amino-acid polypeptide. PTH is secreted by the parathyroid gland and plays a fundamental role in calcium homeostasis as well as increases osteoclast-mediated calcium release from bone, distal renal tubular calcium reabsorption, and intestinal calcium absorption. PTHrP (parathyroid hormone related peptide) is produced by many different tissues and like PTH, PTHrP stimulates bone resorption and renal tubular calcium reabsorption, but in contrast to PTH, PTHrP plays a minor role in intestinal calcium absorption.
Abaloparatide [PTHrP (1-34)] is a 34-amino acid synthetic analog of PTHrP. It was constructed and created to retain stability and therefore, it was hypothesized that abaloparatide would have a more pronounced anabolic action on bone compared with teriparatide. Abaloparatide was approved by the US Food and Drug Administration (FDA) in 2017, under the brand name "Tymlos".
| * Patients with severe osteoporosis or high risk of fracture |
| * Patients who have fractures despite prolonged bisphosphonate use or failure of alternative osteoporosis agents while adherent to treatment |
| * Patients with a very high risk of fractures and low bone formation (for example, T-score less than -3.5 and low markers of bone turnover) |
Currently abaloparatide is FDA approved only for women. Studies in men are in progress. In addition, patients on long-term corticosteroid therapy, and other patients with osteoporosis are needed to determine the full spectrum for use of abaloparatide in osteoporosis.
Abaloparatide therapy should be avoided in:
The contraindications are the same as teriparatide. In addition, this medicine has not been studied in patients with liver disease, and it is not known if the anabolic effects of abaloparatide will work. The drug has not been studied in those with later stages of chronic kidney disease, nor in those with kidney stones. Thus, we do not use either teriparatide or abaloparatide in the following cases:
80 µg daily subcutaneous injection in periumbilical region. Available in prefilled pen, which does not require refrigeration between doses. Studies of the related PTH 1-84 show a difference between thigh and abdomen sites, that the thigh sites provided slower absorption than abdomen due to uptake by the lymphatic system (Tay,). This may be the reason that the abdominal site was used in studies of abaloparatide.
18 months duration
Following this, the benefits of anabolics are "consolidated" when antiresorptive agents are administered after abaloparatide (Cosman). Otherwise there will be a prompt and irreversible loss of bone volume as the bone resorption remains high but the bone formation is no longer active.
There is no long-term safety data with abaloparatide. Hypercalcemia and hypercalciuria are the two most common side-effects, although clinically substantial elevations rarely occur.
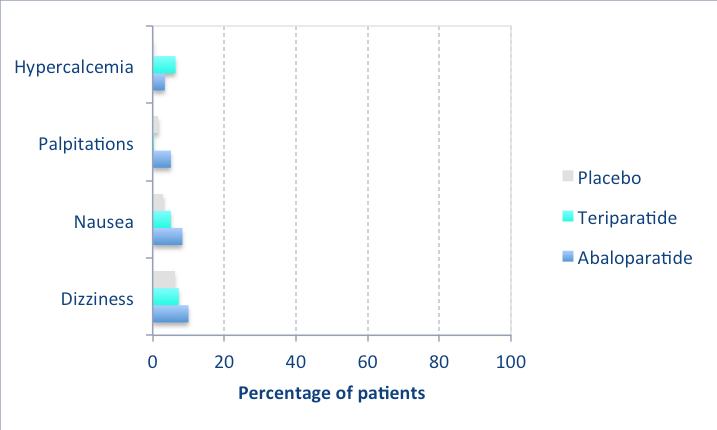
The following graph shows the side effects seen in the clinical trial (Miller), compared to placebo and teriparatide, when there was more than 3% difference between groups. Most were not serious and did not cause discontinuation of the medication. As shown, none were seen in more than 10% of the patients:
A dose-ranging study of 222 women, lasting 6 months, demonstrated that abaloparatide improved bone density (Leder, 2015). This led the the major trial of 2436 women(Miller) with several extensions (ACTIVE phase 2,3, and ACTIVExtend). The ACTIVE phase 2 and phase 3 trials indicated a reduction of risk of vertebral and non-vertebral fractures until 18 months of treatment; it is not clear how long the benefit associated with abaloparatide may endure.
In summary, abaloparatide is more effective than placebo and at least as effective as teriparatide in increasing BMD and decreasing fracture risk for both vertebral and nonvertebral fractures in postmenopausal osteoporosis. Sequence of abaloparatide followed by alendronate can provide a substantial and sustained favorable effect.
Abaloparatide has not been given at the same time as other osteoporosis medications. However, like teriparatide, the bone density decreases rapidly after the medication is discontinued and it's important to plan on switching to an antiresorptive medication. Also, since patients develop rapid bone loss when switched from denosumab to teriparatide, it would probably also be seen with abaloparatide and this should be avoided.
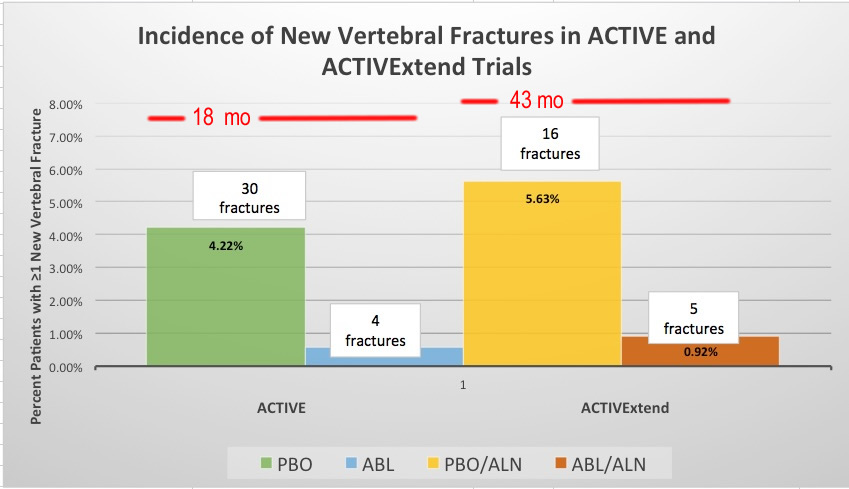
This graph presents data from ACTIVE and ACTIVExtend on the percentage and number of new vertebral fractures with placebo (PBO) or Abaloparatide (ABL) for 18 months and then Placebo followed by Aledronate (PBO/ALN) or Abaloparatide followed by Alendronate (ABL/ALN) for 43 months total. This shows fewer new vertebral fractures occurred with abaloparatide than with alendronate. Data from study by Bone, 2018. The investigators have not done studies of abaloparatide followed by placebo because the drug is similar to teriparatide, in which bone is lost again when the drug stopped without switching to a antiresorptive medicine.