Calcitonin
Calcitonin, produced by cells in the thyroid gland, acts directly on osteoclasts (via receptors on the cell surface for calcitonin). The osteoclasts shrink and stop bone resorption. Bone biopsies from patients treated with the drug show no effects on mineralization. It has a short half-life.
 March 2013: A scientific advisory committee voted (by a narrow margin) to restrict use of calcitonin due to a slight increased risk of cancer when results from all the calcitonin studies were combined. Noel Weiss, an epidemiologist from the University of Washington, was asked to put these findings into context and he stated that "the nature of the available data provide no more than a hint that an increase in risk might be present." Here are some of the presentations.
Here is a link to background information prepared by FDA staff. The FDA will consider this issue later this year.
March 2013: A scientific advisory committee voted (by a narrow margin) to restrict use of calcitonin due to a slight increased risk of cancer when results from all the calcitonin studies were combined. Noel Weiss, an epidemiologist from the University of Washington, was asked to put these findings into context and he stated that "the nature of the available data provide no more than a hint that an increase in risk might be present." Here are some of the presentations.
Here is a link to background information prepared by FDA staff. The FDA will consider this issue later this year.
Indications
Prevention of vertebral compression fractures
Dose
Nasal spray, 200 units/day
Side effects
Minor adverse effects such as nasal irritation are seen in a small number of patients. Calcitonin does not reduce the serum calcium levels below normal in patients with postmenopausal osteoporosis. It may reduce the magnesium in some cases.
This natural hormone has been in clinical use for many years with a very good safety profile.
Effects on bone
A clinical trial of calcitonin enrolled 1255 women with postmenopausal osteoporosis, in whom 975 had vertebral fractures. 783 completed 3 years and 511 completed 5 years. At least one followup xray was done in about 270 women per treatment group: placebo, 100, 200 or 400 units of calcitonin.
Vertebral fractures in calcitonin study
% of women with at least one new fracture
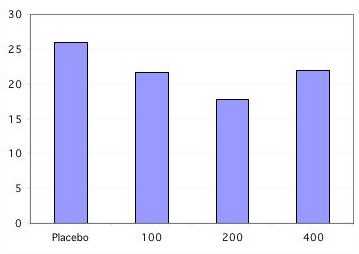
Only the 200 unit dose caused significant reductions in fractures. Bone density by DEXA increased only about 1-2%, and serum markers of bone resorption decreased moderately. There were no significant differences in non-vertebral fracture rates in this study
(Chesnut, C. H., 3rd).
Updated 3/25/13


 March 2013: A scientific advisory committee voted (by a narrow margin) to restrict use of calcitonin due to a slight increased risk of cancer when results from all the calcitonin studies were combined. Noel Weiss, an epidemiologist from the University of Washington, was asked to put these findings into context and he stated that "the nature of the available data provide no more than a hint that an increase in risk might be present." Here are some of the presentations.
Here is a link to background information prepared by FDA staff. The FDA will consider this issue later this year.
March 2013: A scientific advisory committee voted (by a narrow margin) to restrict use of calcitonin due to a slight increased risk of cancer when results from all the calcitonin studies were combined. Noel Weiss, an epidemiologist from the University of Washington, was asked to put these findings into context and he stated that "the nature of the available data provide no more than a hint that an increase in risk might be present." Here are some of the presentations.
Here is a link to background information prepared by FDA staff. The FDA will consider this issue later this year.

