More about the FLEX study
Original analysis
The original report of the FLEX trial was published in 2006 in JAMA by Black DM.
There were 437 women on placebo and 653 on alendronate. Those who had hip T-score lower than -3.5 or who did not increase the hip BMD after the first 5 yrs. of alendronate were excluded. The results showed that there were no differences in morphometric vertebral fractures or in non-vertebral fractures between the groups. The only difference seen was a lower incidence of clinically recognized vertebral fractures in the alendronate groups. (of note, in all other studies that I know about, the morphometric fracture rate is considered the "gold standard" for efficacy). This was shown in the graph on the previous page.
Original analysis looking at the 322 women with T-score less than -2.5
The investigators did a post-hoc analysis looking at baseline bone density and reported that:
"The post hoc subgroup fracture analysis did not show significant trends with lower BMD or prevalent vertebral fractures at FLEX baseline for either nonvertebral or clinical vertebral fractures."
There were 322 women with T-score less than -2.5 and in those women 29.5% on placebo had a nonvertebral fracture compared to 22.6% of the women taking alendronate. Clinical vertebral fractures were seen in 8.3 and 4.7%, respectively. Neither of these were significant.
Later analysis emphasized the 184 women with T-score less than -2.5
who also did NOT have a vertebral fracture at the beginning of the study
 ERROR discovered 1/25/11: I combined the percentages for the subjects with osteopenia on the previous graph and did not do it correctly (just added percentages so they were all twice as high, but the relative risks were still correct). Shown now are the corrected numbers without combining the groups.
ERROR discovered 1/25/11: I combined the percentages for the subjects with osteopenia on the previous graph and did not do it correctly (just added percentages so they were all twice as high, but the relative risks were still correct). Shown now are the corrected numbers without combining the groups.
They then did another post-hoc analysis, reported by Schwartz AV in 2010. This time there were six baseline categories based on low hip T-score (less than -2.5, between -2 and -2.5, and greater than -2) and vertebral fracture at the beginning of the FLEX study, with results as follows:
Fractures during the 5-year FLEX study
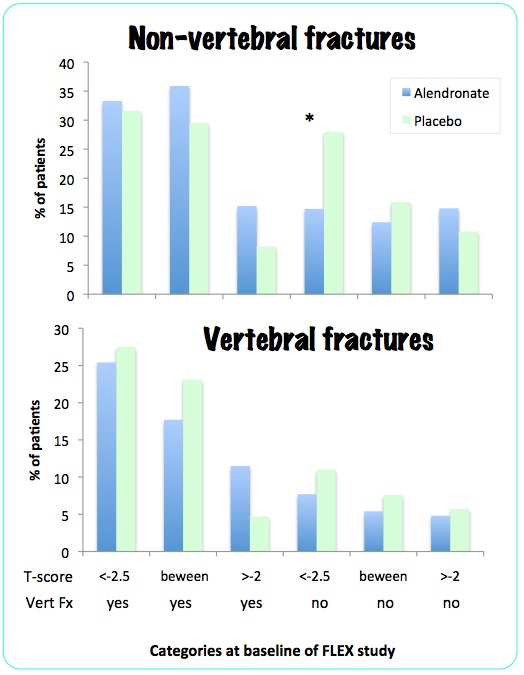
Of all the comparisons, only the non-vertebral fractures in the osteoporotic patients without a prevalent fracture showed a statistically significant difference.
In summary:
|
In the FLEX study there was no reduction in morphometric vertebral fractures in any group. There was overall no reduction in non-vertebral fractures. In the 322 women with bone density T-score less than -2.5 there was no reduction in fracture risk. In a sub-group post-hoc analysis, 184 women with osteoporosis but without vertebral fractures had a reduction in non-vertebral fractures.
|
Reports in the literature that emphasize only the positive results
However, two recent editorial reviews have reported this in a way
that suggests more benefit than was actually seen:
Watts NB:
"At the
end of the Fracture Intervention Trial Long-Term Extension
(FLEX), 5-yr fracture rates for new vertebral fractures were
reduced by 55% in the subjects who had 10 yr of treatment
compared with those who had 5 yr on/5 yr off. Although the
published report suggested no differences in radiographic
vertebral fractures or nonvertebral fractures, a subsequent
analysis indicated that, among subjects with T-scores of -2.5 or below, nonvertebral fracture risk was reduced by
50%"
Shane E:
". . . the use of alendronate for 10 years, as compared with 5 years, was associated with significantly fewer new vertebral fractures and nonvertebral fractures in patients with a bone mineral density T score of -2.5 of below."
Updated 1/25/11


 ERROR discovered 1/25/11: I combined the percentages for the subjects with osteopenia on the previous graph and did not do it correctly (just added percentages so they were all twice as high, but the relative risks were still correct). Shown now are the corrected numbers without combining the groups.
ERROR discovered 1/25/11: I combined the percentages for the subjects with osteopenia on the previous graph and did not do it correctly (just added percentages so they were all twice as high, but the relative risks were still correct). Shown now are the corrected numbers without combining the groups.

