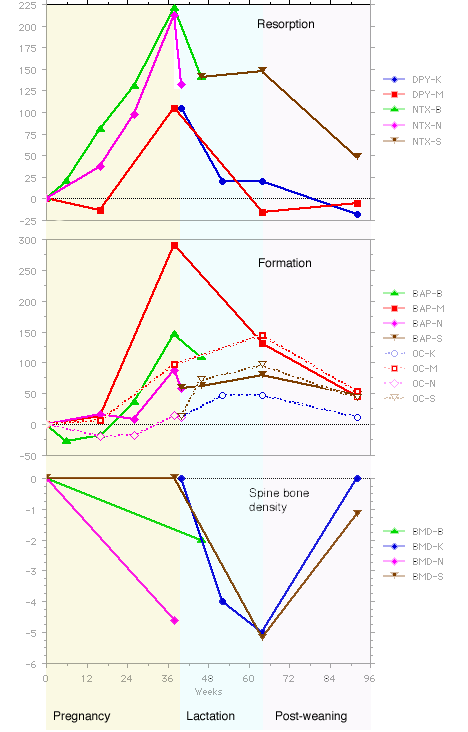
A longitudinal study of bone mineral density in a primate model. This graph shows the bone density in the spine, measured by DEXA and adjusted for 3 dimensions to avoid the size artifact. These were young animals who were still growing (analagous to adolescent humans). During pregnancy (light yellow) the bone density stopped gaining and began to show a loss. Lactation (light blue) was associated with a significant drop in the bone density, which promptly recovered after weaning. Bone biopsies were done at mid-pregnancy and weaning, demonstrating a reduced bone formation rate at mid-pregnancy and an increased bone formation at weaning. Labels: -6 mo, -3 mo: months prior to conception; early: early pregnancy; mid: mid-pregnancy; part: parturition; lact: lactation; post: post-weaning. [from Ott et al]

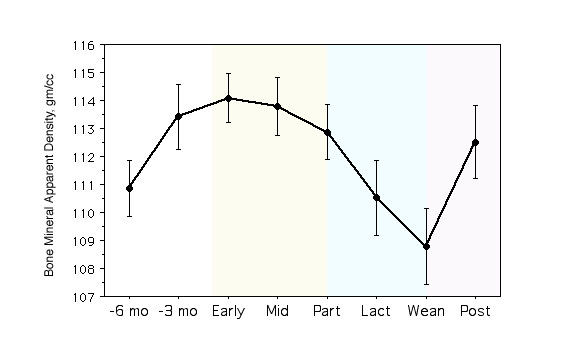
These graphs show several longitudinal studies of human pregnancy and lactation. The data have all been converted to percent change from baseline. In those studies that began after delivery, the baseline from one of the studies of pregnancy was used. Top graph: markers of bone resorption (dpy: deoxypyridinoline; NTX: N-telopeptide). During the first trimester of pregnancy the bone resorption increased a small amount, increasing to very high levels during the last trimester. The resorption remained high during early lactation and then started to decline. After weaning resorption returns to baseline levels. The middle graph shows markers of bone formation. BAP = bone alkaline phosphatase, OC = osteocalcin. These values are unchanged or depressed during the first half of pregnancy, and then start to increase. The data on bone alkaline phosphatase during lactation is variable, but all studies show a return towards baseline following weaning. The bottom panel shows bone density of the spine by DEXA. Studies during pregnancy are variable, but losses during lactation approach 1% per month. As in the primates, rapid recovery is seen after weaning. [data from studies by Black, Sowers, Kalikwarf, More and Naylor]
| Bisphosphonates should be avoided during pregnancy. |
|---|
Rats and rabbits given pamidronate at daily doses 10 times highter than recommended human dose. Result: reduced viable pups, reduced serum calcium in mothers, protracted parturition, pups had shortened long bones (Graepel).
Rats given high doses alendronate - 10mg/kg/day. Result: protracted deliveries, neonatal deaths, maternal hypocalcemia. Fetuses were normocalcemic. (Minsker)
Mice given risedronate did not demonstrate placental transfer but the bones from the neonates of animals given risedronate had abnormalities in tissue culture (Richardson).
Toxicology reviews reference preclinical studies by Sugawara (journal not in Medline) which show abnormal dental eruption and problems with growth and development in rats treated with the highest doses.
Rats given alendronate. Results: Fetuses showed increased amount of bone and decreased bone marrow. 14C alendronate passed through the rat placenta and accumulated in the fetuses (Patlas).
Bone scan agent (methylene diphosphonate) uptake seen in fetal skeleton in mothers with malignant tumors (McKenzie).
Pamidronate given to one woman who was lactating, and breast milk did not have pamidronate identified (Siminoski).
One baby with no serious abnormalities when mother given pamidronate for breast cancer (Illidge).
One woman given alendronate, apparently no problem with triplet babies, but article in German and abstract has very little information about timing of the medication or details about the infant (Harsch).
One woman who took oral pamidronate had an infant without obvious birth defects. However, the infant weighed only 8kg (10th percentile) at one year (Rutgers-Verhage).
Study of alendronate prescriptions in England. Of 11,916 patients, only one was pregnant, and there were no obvious problems with the baby. One other woman had taken alendronate prior to pregnancy without reported problems (Biswas).
Two young women with osteogenesis imperfecta who had previously received pamidronate became pregnant. The babies also had osteogenesis imperfecta. One was hypocalcemic as a neonate, the other had bilateral talipes equinovarus. No abnormalities in the skeletal modeling of the infants were found (Munns).
The above are all the studies I could find in Medline. I also tried searching with Google and found one other report, which I can't verify but include it only because there is so little information about this topic:
"Ange" said "we are aware of 3 or 4 cases of young women who unintentially
became pregnant while on pamidronate. Each one had been receiving the drug for a
different period of time and all of them stopped the treatments as soon as
they were aware that they were pregnant. They were all participants in
research studies. " 1/13/04, www.talkaboutsupport.com
The bisphosphonates used to be in FDA category "C" but now some of them are in FDA category "D" . Here is a copy of the 2004 FDA label information for pamidronate:
Pregnancy Category D (See WARNINGS)
There are no adequate and well-controlled studies in pregnant women.
Bolus intravenous studies conducted in rats and rabbits determined that Aredia produces
maternal toxicity and embryo/fetal effects when given during organogenesis at doses of 0.6 to
8.3 times the highest recommended human dose for a single intravenous infusion. As it has
been shown that Aredia can cross the placenta in rats and has produced marked maternal
and nonteratogenic embryo/fetal effects in rats and rabbits, it should not be given to women
during pregnancy.
Bisphosphonates are incorporated into the bone matrix, from where they are gradually
released over periods of weeks to years. The extent of bisphosphonate incorporation into
adult bone, and hence, the amount available for release back into the systemic circulation, is
directly related to the total dose and duration of bisphosphonate use. Although there are no
data on fetal risk in humans, bisphosphonates do cause fetal harm in animals, and animal
data suggest that uptake of bisphosphonates into fetal bone is greater than into maternal
bone. Therefore, there is a theoretical risk of fetal harm (e.g., skeletal and other abnormalities)
if a woman becomes pregnant after completing a course of bisphosphonate therapy. The
impact of variables such as time between cessation of bisphosphonate therapy to conception,
the particular bisphosphonate used, and the route of administration (intravenous versus oral)
on this risk has not been established.
Nursing Mothers
It is not known whether Aredia is excreted in human milk. Because many drugs are excreted
in human milk, caution should be exercised when Aredia is administered to a nursing woman.
Women who are pregnant should not take bisphosphonates. If women are already taking a bisphosphonate and want to become pregnant, it is not clear how long they should wait. Until more information is available, I suggest waiting at least one year after stopping the medication to try getting pregnant. If a woman is taking a bisphosphonate and inadvertantly gets pregnant and wants to continue the pregnancy, she should be carefully followed, with measurements of calcium and optimal vitamin D levels. Calcitonin is a safe drug to use during pregnancy if bone loss is a concern. Young women should not be getting bisphosphonates anyway unless they definitely have established osteoporosis (that means they have non-traumatic fractures) and other methods are not working. Osteopenia in a healthy woman is not an indication for any bisphosphonate!
Normal
Bezerra, F. F.(2002). Pregnancy and lactation affect markers of calcium and bone metabolism differently in adolescent and adult women with low calcium intakes. J Nutr 132: 2183-7.
Black, A. J.(2000). A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res 15: 557-63.
Holmberg-Marttila, D.(2003). Bone turnover markers during lactation, postpartum amenorrhea and resumption of menses. Osteoporos Int 14: 103-9. Epub 2003 Feb 12.
Kalkwarf, H. J.(1999). Effects of calcium supplementation on calcium homeostasis and bone turnover in lactating women. J Clin Endocrinol Metab 84: 464-70.
More, C.(2003). The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol 106: 209-13.
Naylor, K. E.(2000). The effect of pregnancy on bone density and bone turnover. J Bone Miner Res 15: 129-37.
Ott, S. M.(1999). Bone physiology during pregnancy and lactation in young macaques. J Bone Miner Res 14: 1779-88.
Sowers, M.(1991). A prospective evaluation of bone mineral change in pregnancy. Obstet Gynecol 77: 841-5.
Sowers, M.(1995). Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab 80: 2210-6.
Sowers, M.(1993). Changes in bone density with lactation. Jama 269: 3130-5.
Uemura, H.(2002). Serum osteoprotegerin/osteoclastogenesis-inhibitory factor during pregnancy and lactation and the relationship with calcium-regulating hormones and bone turnover markers. J Endocrinol 174: 353-9.
Bisphosphonate toxicity
Sakiyama, Y.(1986). The effect of ethane-1-hydroxy-1, 1-diphosphonate (EHDP) on fetal mice during pregnancy. Part 2. External anomalies. J Osaka Dent Univ 20: 91-100.
Graepel, P.(1992). Reproduction toxicity studies with pamidronate. Arzneimittelforschung 42: 654-67.
Richardson, A. C.(1993). Risedronate activity in the fetal and neonatal mouse. Otolaryngol Head Neck Surg 109: 623-33.
Minsker, D. H.(1993). Effects of the bisphosphonate, alendronate, on parturition in the rat. Toxicol Appl Pharmacol 121: 217-23.
McKenzie, A. F.(1994). Technetium-99m-methylene diphosphonate uptake in the fetal skeleton at 30 weeks gestation. J Nucl Med 35: 1338-41.
Illidge, T. M.(1996). Malignant hypercalcaemia in pregnancy and antenatal administration of intravenous pamidronate. Clin Oncol (R Coll Radiol) 8: 257-8.
Patlas, N.(1999). Transplacental effects of bisphosphonates on fetal skeletal ossification and mineralization in rats. Teratology 60: 68-73.
Siminoski, K.(2000). Intravenous pamidronate for treatment of reflex sympathetic dystrophy during breast feeding. J Bone Miner Res 15: 2052-5.
Harsch, I. A.(2001). [Osteoporosis and multiple pregnancy--a case report with positive outcome]. Med Klin (Munich) 96: 402-7.
Biswas, P. N.(2003). Pharmacovigilance study of alendronate in England. Osteoporos Int 14: 507-14. Epub 2003 Apr 23.
Schapira, D.(2003). Severe transient osteoporosis of the hip during pregnancy. Successful treatment with intravenous biphosphonates. Clin Exp Rheumatol 21: 107-10.
French, A. E.(2003). Taking bisphosphonates during pregnancy. Can Fam Physician 49: 1281-2.
Rutgers-Verhage, A. R.(2003). No effects of bisphosphonates on the human fetus. Birth Defects Res Part A Clin Mol Teratol 67: 203-4.
Munns, C. F.(2004). Maternal and fetal outcome after long-term pamidronate treatment before conception: a report of two cases. J Bone Miner Res 19: 1742-5. Epub 2004 Jul 21.
Updated 10/21/04
