Diabetes mellitus is a
disorder of blood glucose regulation, which results from a
deficiency in the action of the hormone insulin.
In type 1 diabetes mellitus (T1DM) there is an absolute
deficiency of insulin secretion due to autoimmune
destruction of the insulin-secreting beta
cells in the endocrine pancreas. In type 2 diabetes mellitus (T2DM), there is a decreased
responsiveness of tissues to insulin, known as insulin resistance. T2DM
usually also involves a defect in insulin secretion, but there are
still functioning beta cells. With either type of diabetes,
the result is hyperglycemia,
or high levels of glucose in the plasma.
The goal in treatments for diabetes mellitus is prevention of
hyperglycemia, or glycemic control.
Hyperglycemia may not cause any symptoms, although in some cases
it may cause excessive urination (polyuria) and increased thirst,
as we discussed in fall quarter (see Polyuria
in Diabetes Mellitus). Chronic hyperglycemia causes
various biochemical changes. In particular, chronic
hyperglycemia causes glycation,
which is the non-enzymatic addition of sugars to proteins.
Ultimately, the various chemical changes due to hyperglycemia are
pathological for certain tissues and diabetic
complications ensue. The major diabetic
complications are listed below.
Cardiovascular Disease (heart attack,
stroke, peripheral vascular disease)
Nephropathy (kidney damage)
Retinopathy (blindness)
Peripheral Neuropathy (loss of sensation,
autonomic dysfunction)
Foot Ulcers (amputation)
Several large prospective studies have demonstrated that diabetic
complications can be significantly reduced by effective glycemic
control. An unfortunate adverse effect of more intensive diabetic
therapy is that it increases the risk for hypoglycemia.
Hypoglycemia
(low plasma glucose) is dangerous because the brain needs glucose
for proper functioning. Hypoglycemia causes confusion and
sometimes loss of consciousness.
Glycemic control can be determined through frequent monitoring of blood glucose, but in practice it is determined by measuring HbA1c, or the percentage of glycated hemoglobin. Hyperglycemia causes glycation of proteins, and hemoglobin is a convenient protein to examine since it can be obtained from a simple blood draw. HbA1c (expressed as a percentage) reflects the degree of glycemic control in the previous 4-8 weeks. The American Diabetes Association recommends an HbA1c target of less than 7% for most diabetics. Researchers look at how much the HbA1c is lowered to determine the effectiveness of treatments for diabetes mellitus. HbA1c is also now used as a tool for diagnosing diabetes mellitus.
Below are listed major drug treatments for diabetes mellitus that we will discuss in PBIO 376 quiz section.
Insulin therapy is necessary
for type 1 diabetics because they have an absolute insulin
deficiency due to the autoimmune destruction of the pancreatic
beta cells. In fact, an older name for T1DM was "insulin-dependent
diabetes mellitus", reflecting the need for insulin
treatment. Insulin therapy is also used in treating T2DM. As
T2DM advances, the beta cells become damaged by hyperglycemia and
significant defects in insulin secretion develop, causing some
type 2 diabetics to require insulin. A big problem with
insulin therapy is the risk of hypoglycemia,
which occurs when insulin levels are too high.
Metformin is the most widely
prescribed drug for treating T2DM. It is the first drug of choice
to treat newly diagnosed diabetes, and also is sometimes used to
treat pre-diabetes. The mechanism of action of metformin is
complicated and is still not entirely understood. It has
several effects that are helpful in the treatment of T2DM:
it lowers glucose production in the liver, it sensitizes tissues
to insulin, and it promotes weight loss. The majority of
diabetics with T2DM are overweight. Excess adipose tissue
(as occurs when people are overweight) is linked to insulin
resistance so weight loss is helpful in the treatment of T2DM.
To understand how sulfonylureas and meglitinides work, you need to understand the mechanism of insulin secretion in pancreatic beta cells. This is outlined in Figure 5.26 on p. 158 of the textbook. These drugs bind to and close KATP, the ATP-sensitive K+ channel on pancreatic beta cells. Closing K+ channels causes depolarization, allowing Ca++ entry to stimulate insulin secretion.
These drugs improve glycemic control, but patients taking them tend to gain weight. Sulfonylureas (glipizide, glyburide, glimepiride) are older drugs and less expensive. A potential problem is that they can induce too much insulin secretion and hypoglycemia can result. The meglitinides (repaglinide, nateglinide) are newer drugs that are designed to avoid this problem. They have a shorter half-life, and are taken at mealtimes to enhance insulin secretion and prevent postprandial hyperglycemia ("postprandial" means after eating).
Incretins are gastrointestinal hormones that increase glucose-dependent insulin secretion. There are two main incretin hormones in humans, GIP (glucose-dependent insulinotropic peptide; also known as gastric inhibitory peptide) and GLP-1 (glucagon-like peptide-1). Both hormones are polypeptide hormones secreted by enteroendocrine cells that are located in the epithelium of the small intestine.
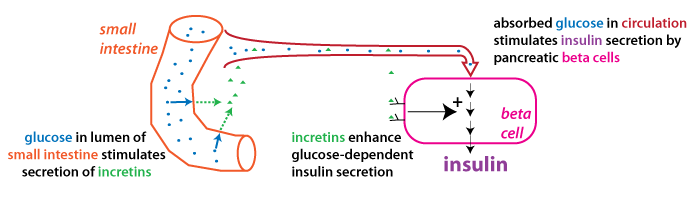
The mechanism of incretin action is schematized in the figure
below. Glucose in the small intestine stimulates incretin release.
Incretins are carried through the circulation to their target
tissue: the pancreatic beta cells.
Incretins enhance glucose-dependint
insulin secretion: this means they
cause beta cells to secrete more insulin in response to
the same amount of blood glucose. This is a feedforward effect in that the
enhanced insulin secretion stimulated via the incretins
anticipates the rise in blood glucose that occurs after glucose in
the small intestine gets absorbed.

There are two types of incretin-based drugs that are used to
treat T2DM. GLP-1 agonists
are peptide drugs with a longer half-life than the native hormone
because they are resistant to degradation by dipeptidyl
peptidase-4 (DPP-4),
the main protease that breaks down GIP and GLP-1. Since they
are peptides, they must be injected (but see below). DPP-4 inhibitors ("gliptins")
prolong the action of native incretins. DPP-4 inhibitors are
less effective at lowering HbA1c than GLP-1 agonists, but an
advantage is that they are oral drugs.
GLP-1 agonists have the added benefit of inducing
weight loss.
Depending on the drug, weight loss ranges from 5-20% of body
weight. One potential mechanism may be that GLP-1 delays
stomach emptying into the small intestine, causing patients
to eat less because they feel “full” sooner. GLP-1 also may
have effects in the brain that promote satiety. Two GLP-1
receptor agonist drugs (liraglutide and semaglutide) have FDA
approval for treatment of overweight/obesity independent of
diabetes mellitus. Tirzepatide (a dual GIP/GLP-1 receptor
agonist) is another diabetes drug that was approved in 2023 as an
overweight/obesity treatment. Weight loss in the phase III
trial of tirzepatide was greater than 15% in most study
participants.
In 2019, the FDA approved the first oral
GLP-1 agonist (oral semaglutide, Rybelsus™).
Semaglutide is linked to a molecule called salcaprozate sodium or
SNAC that protects it from digestion in the stomach and also aids
its transport across the gastric epithelium. The oral
formulation of semaglutide is just as effective at lowering HbA1c
as the subcutaneous drug and appears to have the same benefits
(promotes weight loss, reduces the risk for cardiovascular
disease).
SGLT2 inhibitors are drugs
that reduce glucose reabsorption in the kidney by blocking the
protein SGLT2, the main sodium-glucose cotransporter expressed in
the kidney tubules. These drugs all have the suffix "-gliflozin"
in their name. SGLT2 inhibitors have been shown to improve
glycemic control and cause weight loss, since glucose lost in the
urine is calories lost from the body. SGLT2 inhibitors also have a
modest effect to lower blood pressure.
SGLT2 inhibitors are important new drugs for the treatment of T2DM. They are oral drugs, and so are easy for patients to use. Most importantly, several large trials have shown that treatment with SGLT2 inhibitors reduces the risk for cardiovascular and kidney disease, two major complications of diabetes mellitus.
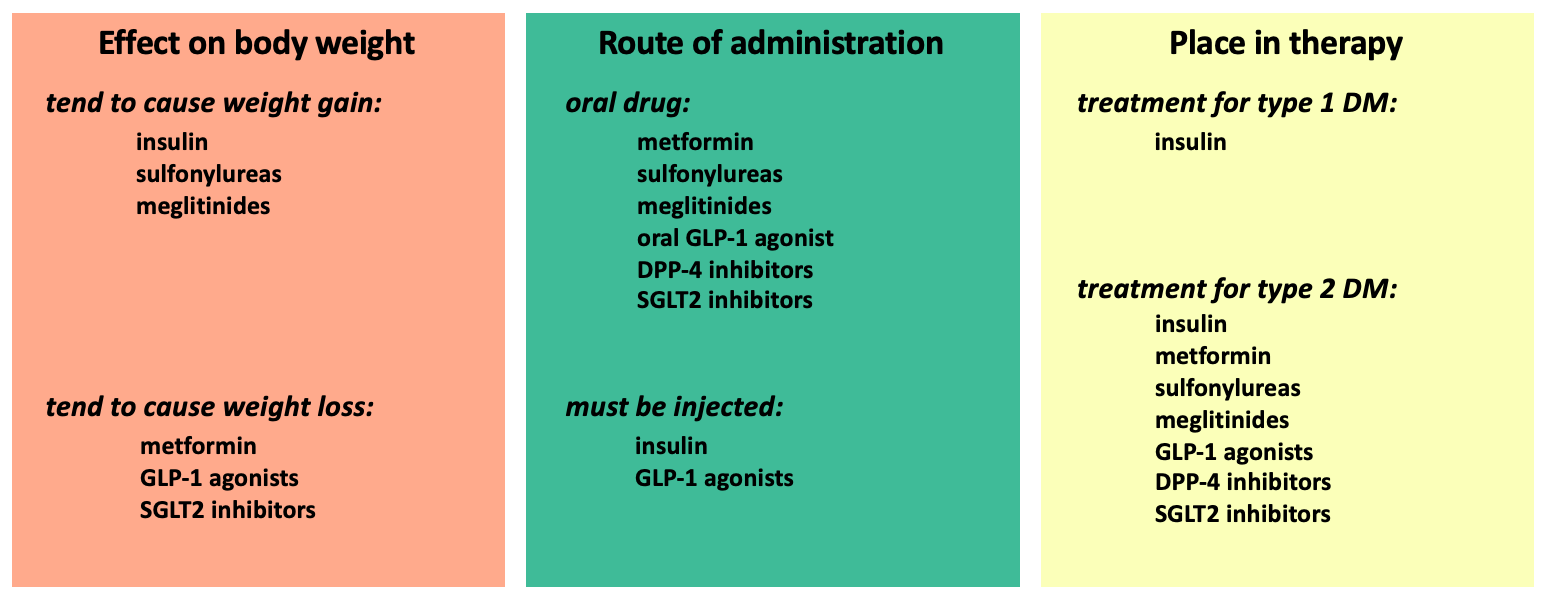
In the discussion above, the drugs for diabetes mellitus are discussed according to their mechanism of action. In the tables below, the drugs are organized according to other factors that may be important in guiding treatment decisions.
Two widely used drug treatments not included on this page are the thiazolidinediones (piglitazone and rosiglitazone) and the peptide drug pramlintide.