

| TERIPARATIDE |
Parathyroid hormone is naturally an 84-amino-acid polypeptide. In 2002 the FDA approved recombinant PTH 1-34, chemical name teriparatide made by Lilly with the brand name Forteo. In 2017 a related drug called abaloparatide was also approved, brand name Tymlos. Teriparatide and abaloparatide are ANABOLIC medications that reduce the incidence of osteoporotic fractures. Anabolic drugs work by increasing formation of new bone, whereas other drugs merely block the resorption of bone.
| * Patients with severe osteoporosis and vertebral compression fractures |
| * Patients who have fractures despite prolonged bisphosphonate use |
| * Patients with a very high risk of fractures and low bone formation (for example, T-score less than -3.5 and low markers of bone turnover) |
| * Prolonged use of high dose prednisone in patient with osteoporosis |
I also consider teriparatide in the following situations, based on animal studies and some clinical observations:
|
WARNING In male and female rats, teriparatide caused an increase in the incidence of osteosarcoma (a malignant bone tumor) that was dependent on dose and treatment duration. The effect was observed at systemic exposures to teriparatide ranging from 3 to 60 times the exposure in humans given a 20-mcg dose. Because of the uncertain relevance of the rat osteosarcoma finding to humans, teriparatide should be prescribed only to patients for whom the potential benefits are considered to outweigh the potential risk. Teriparatide should not be prescribed for patients who are at increased baseline risk for osteosarcoma (including those with Paget's disease of bone or unexplained elevations of alkaline phosphatase, open epiphyses, or prior external beam or implant radiation therapy involving the skeleton) |
Teriparatide therapy should be avoided in:
In addition, this medicine has not been studied in patients with liver disease, and since liver enzymes can break down PTH, it is not known if the anabolic effects of PTH will work. The drug has not been studied in those with later stages of chronic kidney disease, nor in those with kidney stones. Thus, I do not use teriparatide in the following cases:
The available dose is 20 mcg/day, given by intermittent subcutaneous injection once a day using a special injection device. It is easy to use, the needle is very tiny, and the injection is not painful.
The duration of treatment should be 18 to 24 months. The anabolic actions start to fade after about 12 to 18 months (Finkelstein JS, Leder BZ.) Lindsay R reported a decreased risk of fractures with duration of therapy up to 24 months.
 After treatment with teriparatide, patients should be treated for the next two years with an anti-resorbing medication; otherwise the bone density will decrease. (Black DM, Kurland ES,
Rittmaster R, Prince R, Leder BZ, Eastell R)
After treatment with teriparatide, patients should be treated for the next two years with an anti-resorbing medication; otherwise the bone density will decrease. (Black DM, Kurland ES,
Rittmaster R, Prince R, Leder BZ, Eastell R)
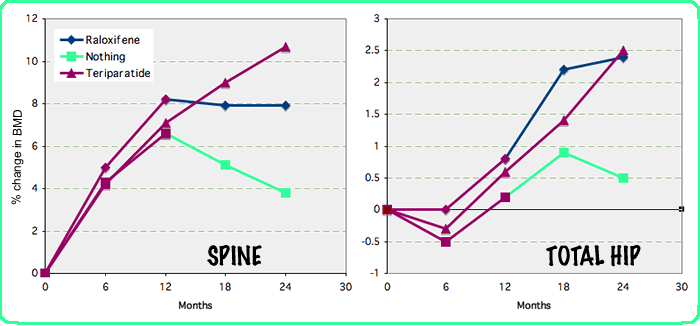 This graph presents data from a recent study by Eastell, in which 507 patients were given teriparatide for a year and then randomly treated with teriparatide, raloxifene, or no active drug for the next year. All received calcium and vitamin D. This shows the usual increase in the spine, as well as the typical early loss and later gain in cortical bone such as the total hip. The bone density decreased when teriparatide was stopped and remained stable at the spine with raloxifene. Similar results are seen when bisphosphonates are given after the teriparatide treatment.
This graph presents data from a recent study by Eastell, in which 507 patients were given teriparatide for a year and then randomly treated with teriparatide, raloxifene, or no active drug for the next year. All received calcium and vitamin D. This shows the usual increase in the spine, as well as the typical early loss and later gain in cortical bone such as the total hip. The bone density decreased when teriparatide was stopped and remained stable at the spine with raloxifene. Similar results are seen when bisphosphonates are given after the teriparatide treatment.
Cosman showed that giving PTH1-34 in 3 month cycles (daily for 3 months, off for 3 months) for 15 months gave a similar increase in bone density as daily for 15 months. In a follow-up study a year later (Cosman F) she found that the patients who were retreated showed an increase in the bone density. The patients had been on alendronate at least a year prior to baseline and had remained on alendronate the entire time. Other studies suggest that alendronate will attenuate the response to teriparatide, and this study did not include patients treated with teriparatide alone.
Finkelstein studied a group of patients who were treated with teriparatide for 2 years, then took no medicines for a year, and then were re-treated with teriparatide for a year. The bone density decreased when the teriparatide was discontinued and increased again with the retreatment, but the response the second time was much less than the first time. The markers of bone formation (P1NP) increased by over 1200% the first time and only 200% the second time. This was not caused by development of antibodies. These authors studied a group of patients who were treated with alendronate as well, but did not report their findings.
Osteosarcomas develop in about 50% of rats treated with high doses of intermittent PTH. This has not been shown in dogs or primates (Vahle JL). In humans, the rate is not different from the background incidence rate (one in 250,000). Nevertheless, this drug should not be used in patients with a risk of bone cancer until there has been even more clinical experience.
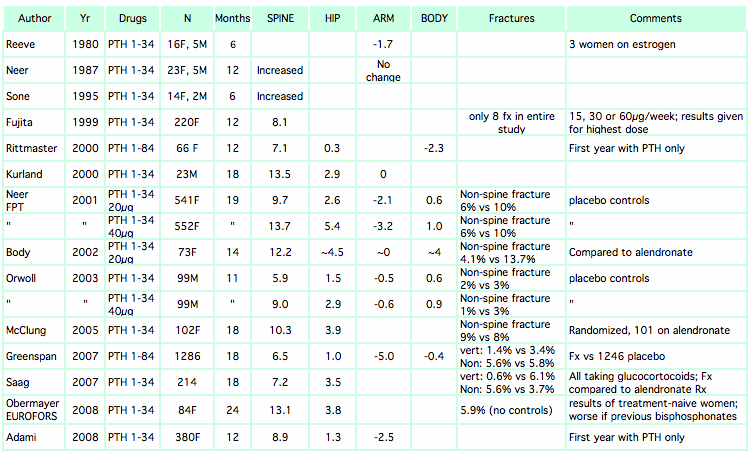
This table shows percent change from baseline at different skeletal sites. Most studies used 1-34 PTH (teriparatide) but a few used 1-84 PTH. You can download the Excel file for these tables.
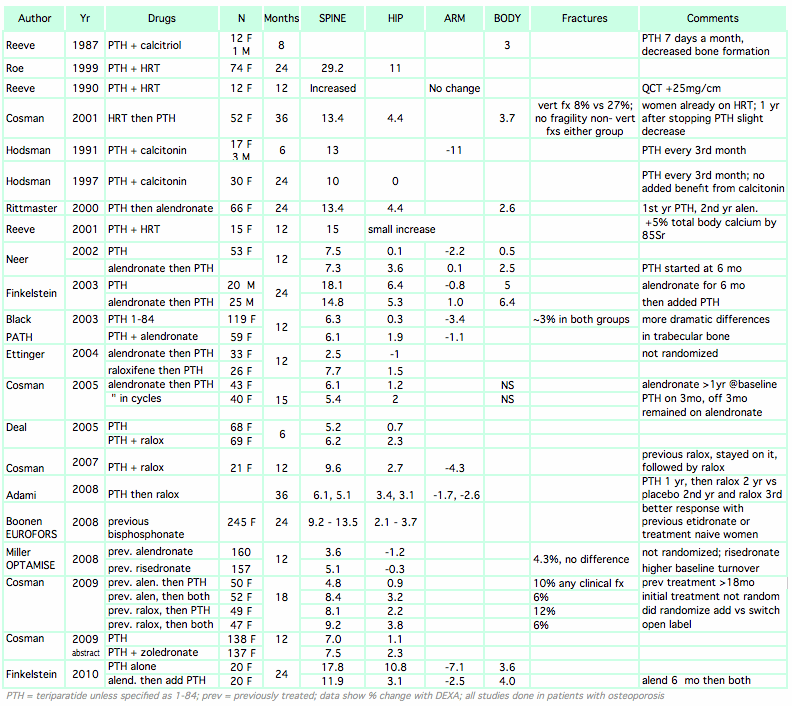
It is logical to combine an anti-resorptive drug with an anabolic drug, but unfortunately that does not work very well and sometimes can be dangerous. The following studies show that:
| * Prior or concurrent treatment with potent bisphosphonates blunts the anabolic response to teriparatide, especially at the spine |
| * However, there is still benefit even after prolonged bisphosphonate use |
| * Concurrent treatment with raloxifene may be slightly better than teriparatide alone |
| * An abstract at the 2009 ASBMR by Cosman found that zoledronic acid combined with teriparatide resulted in higher bone density after one year. There is a possibility that different bisphosphonates will have different effects in combination with teriparatide. |
 Following denosumab, the bone density decreases rapidly and this is not prevented by teriparatide (it could even be worse). Leder, 2015 Following denosumab, the bone density decreases rapidly and this is not prevented by teriparatide (it could even be worse). Leder, 2015 |
A randomized, double-blinded clinical trial of 428 patients with glucocorticoid-induced osteoporosis compared teriparatide to alendronate (Saag KG). The bone density increased 7.2% in the teripartide group and 3.4% in the alendronate group. Furthermore, the vertebral fracture rate was significantly less in the teriparatide treated patients (0.6 vs 6.1%) although the non-vertebral fractures were not better (5.6 vs 3.7%). A post-hoc analysis of the study showed that teriparatide improved bone density and biomarkers of bone formation in subgroups according to age and gender (Langdahl BL).
 Aspenberg P studied 102 postmenopausal women who had a distal radial fracture and randomly treated them with teriparatide (20 or 40 mcg/d) or placebo within 10 days of the fracture. There was no improvement in healing time with the 40 mcg dose. A post-hoc analysis did suggest shorter healing with the lower dose (7.4 vs 9.1 weeks). Another post-hoc analysis found an improvement in early callus formation. Thus, further studies are warranted.
Aspenberg P studied 102 postmenopausal women who had a distal radial fracture and randomly treated them with teriparatide (20 or 40 mcg/d) or placebo within 10 days of the fracture. There was no improvement in healing time with the 40 mcg dose. A post-hoc analysis did suggest shorter healing with the lower dose (7.4 vs 9.1 weeks). Another post-hoc analysis found an improvement in early callus formation. Thus, further studies are warranted.
Chintamaneni S reported a case of a patient with an atrophic sternal nonunion that was causing persistent pain for 6 months. He had no infection or underlying metabolic problem. There was an 8mm gap. After 3 months of teriparatide he demonstrated healing, which was complete by 9 months. Rubery PT described good response in 3 cases of nonunion of type III odontoid fractures.
Alkhiary studied rats and showed that daily subcutaneous administration of low-dose teriparatide, PTH (1-34), enhances fracture healing by increasing BMD, BMC, and strength, and produces a sustained anabolic effect throughout the remodeling phase of fracture repair. Kakar S has also shown improved fracture repair, associated with enhanced chondrogenesis and Wnt signalling. Several clinical trials are addressing this, including one sponsored by the NIH about healing pelvic fractures in Rochester (NCT00594906), and one in Denmark looking at healing of shoulder fractures (NCT00741182).
 Click for more details about teriparatide, bone histology, and physiology.
Click for more details about teriparatide, bone histology, and physiology.

