
Mechanical Ventilation
Case 3 Answers
A patient is admitted to the ICU with severe necrotizing pancreatitis. A few hours after admission, he developed increasing oxygen requirements and was intubated for hypoxemic respiratory failure. Initially, his oxygen saturations improved to the mid-90% range on an FIO2 of 0.5, but in the past 2 hours, the nurse has had to increase the FIO2 back to 0.7 and his SaO2 is still in the lower 90% range. The patient remains on a PEEP of 5 cm H2O. The nurse drew an ABG which shows pH 7.35, pCO2 38, PO2 60, HCO3- 22 on an FIO2 of 0.8. The patient’s repeat chest x-ray is shown below. An echocardiogram performed earlier in the day revealed normal left ventricular function.
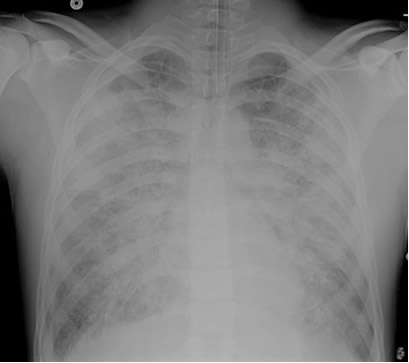
How do you explain his worsening oxygenation status?
The patient’s chest x-ray shows diffuse bilateral opacities in a pattern consistent with pulmonary edema. The ratio of the PaO2 to FIO2, referred to as the P/F ratio, is 75. He also has an echocardiogram showing evidence of normal left ventricular function. Based on the combination of the x-ray findings, the low P/F ratio and the evidence of normal left ventricular function, this patient should be classified as having developed the Acute Respiratory Distress Syndrome (ARDS). Pancreatitis is one of several known precipitating factors for this syndrome. Other known precipitants include pneumonia or other forms of severe infection, trauma, severe burns, aspiration of gastric contents, drug overdose and a variety of other processes.
What can you do to improve his oxygenation?
There are two initial options for improving oxygenation for people on mechanical ventilation: raising the FIO2 and increasing the PEEP (positive end-expiratory pressure). PEEP helps keep the alveoli open and prevents small airway closure. It is very effective in diffuse processes such as ARDS, pulmonary edema or diffuse alveolar hemorrhage. It is generally not effective and may actually worsen oxygenation in focal processes such as lobar pneumonia. In these cases, the PEEP gets preferentially applied to the more compliant normal lung and does not open alveoli in the diseased portions. The normal alveoli may then become over-distended leading to compression of the capillaries running between the alveoli. As a result, blood flow to these normal regions is decreased and blood is shunted to the abnormal areas of the lung where poor ventilation/perfusion (V/Q) matching leads to worsening hypoxemia. In focal processes, increasing the FIO2 may be the best option for improving oxygenation.
It is important to remember that although increasing the PEEP may improve oxygenation in certain cases, it is not an entirely benign intervention. High levels of PEEP (> 15 H2O) can impair venous return and, therefore, decrease cardiac output and blood pressure. Ironically, even though the PaO2 may be improved on the higher level of PEEP, a drop in cardiac output will actually impair oxygen delivery to the tissues. High levels of PEEP also increase the risk of barotrauma. Given the risk of such problems, the clinician must always assess whether the increased level of PEEP is providing benefit to the patient. If the PaO2 fails to improve on higher levels of PEEP, then the PEEP should be returned to lower levels in order to avoid the complications described above.
It is also important to remember that while we tend to focus our attention on the PaO2 and the SaO2, our main concern is whether we are actually able to deliver enough oxygen to the tissues. Although the PaO2 and the SaO2 contribute to oxygen delivery (DO2), other factors, including cardiac output and the hemoglobin concentration play a larger role, as you can see in the following equation:
DO2 = Cardiac Output X [(Hb x 1.34 x SaO2) + 0.003xPaO2]
In the severely hypoxemic patient, improving the PaO2 often has only a small effect on overall oxygen delivery; you can have a larger impact by transfusing blood if they are markedly anemic or by improving cardiac output by, for example, administering IV fluids. Considering these other interventions would be an important part of management for any severely hypoxemic patient.
What other changes should you consider making in the ventilator settings?
Because this patient has developed ARDS, he should be put on what is referred to as a “lung-protective” ventilation strategy, in which the tidal volume is incrementally decreased to 6 ml/kg of his ideal body weight. The goal is to bring the static or plateau pressure below 30 cm HO2 (the concept of static pressure is described further below in another case). If this goal is not reached when the patient’s tidal volume is at 6 ml/kg then the tidal volume can be reduced to as low as 4 ml/kg in an effort to reach the target plateau pressure. This strategy is based on the results of the ARDSnet study which demonstrated that patients with ARDS who were ventilated according to this protocol had improved survival when compared to people with ARDS who were ventilated on tidal volumes of 10-12 ml/kg. The mortality benefit is present even if the plateau pressure was below 30 cm H2O before the tidal volume was decreased to 6 ml/kg.
If his oxygen saturation fails to improve despite being on high levels of support (eg. FIO2 of 1.0 and 20 cm H2O of PEEP), what other options do you have for improving his oxygenation?
Several strategies can be used in patients with what is referred to as “refractory hypoxemia”. They all share a common feature: they can improve oxygenation but have not been shown to improve patient outcomes and, in particular, mortality. In some cases, patients are placed in the prone position using a specially designed bed that facilitates this change in patient position. The theory behind this intervention is that many patients develop atelectasis in dependent lung zones. If blood flow continues to these areas, shunt physiology develops and contributes to hypoxemia. By rotating the person to the prone position, this dependent atelectasis is relieved and there are improvements in ventilation-perfusion matching and, as a result, gas exchange.
Another strategy involves using inhaled pulmonary vasodilator medications (nitric oxide or prostacyclin). If given intravenously, these medications would cause diffuse pulmonary vasodilation which, in turn, would increase blood flow to poorly ventilated areas and worsen oxygenation. By using an inhaled form, however, the medication is delivered to and vasodilation occurs only in those areas of the lung that receive adequate ventilation. This helps improve matching of pulmonary blood flow and ventilation and improves gas exchange. Some clinicians employ what are referred to as recruitment maneuvers in which the lung is held in an inflated position for an extended period of time in an effort to reduce atelectasis and open up previously close regions of the lung.
In other cases, patients are given a bolus and/or drip of a paralytic medication such as vecuronium. The goal of the paralytic agent is to eliminate any muscular activity on the part of the patient and, thereby, decrease oxygen consumption. It also eliminates any patient respiratory effort which might be contributing to dysynchrony with the ventilator and either ineffective ventilation or increased oxygen consumption. Finally, some practitioners place patients on alternative modes of mechanical ventilation such as high frequency oscillatory ventilation.
These strategies are associated with risks and, in the case of prone ventilation and inhaled therapies, are associated with very high cost. In the absence of data supporting a mortality benefit, they should only be used in rare circumstances.
UW School of Medicine : School of Medicine Mission
Copyright and Disclaimer : Credits and Acknowledgements