
|
Click on the question mark to ask the class about oceanography, Glacier Bay, or the cruise.† Your question and answer will appear on the Questions page under Quick Links. |
|
Questions and Answers |
|
Question: I'm really enjoying this blog, so thank you for your research and the information you're providing. The photos are incredible -- especially the 12-knot pilot transfer maneuver! At least the guys hopping over the rail had on flashing locator lights. I'll bet the school kids were pretty impressed with the ship and all of you scientists. What a great experience for them, so good for you to bring them aboard. I read the website article on your trip and it was interesting to learn that Glacier Bay hosts one of the richest cold-water marine environments and has a great diversity of top-level marine predators. What is special about Glacier Bay to have this distinction? What's different about it from many other cold-water bays? Also, this baseline data that you're collecting for the bay will be so important and valuable. Why hasn't research such as this been conducted before? Thank you for your time and your important work. Answer:† Those are great questions and really get at the heart of why we are here.† The short answer to your first question is we donít know!† It is our hope that some of the data we collect on this cruise will shed some light on these issues.† In the next couple of days I will post a blog detailing a conversation that I had with Leuis Sharman, a marine ecologist with Glacier Bay National Park, which will probe this issue in a little more depth.† For now it is probable that the unique tidal and topographical features of Glacier Bay enhance the ice effects that lead to high primary productivity. This leads directly into your second question.† Again, we donít really know (but hope to find outÖ).† The tide regime here is somewhat unique, being not unlike other glaciated west coast fjords but certainly different from east coast ones.† Perhaps these tidal effects are augmented by local topography to create a unique set of characteristics. As to why more research has not been conducted, remoteness probably plays a big role.† There are so many places to study and so few research dollars and research vessels to go around, the more exotic locales can easily miss ever being put on an institutionís itinerary. Additionally, with so many marine environments in serious trouble it may be difficult for institutions to justify the expense of studying one of the few places that is not (of course many scientists disagree with this view, baseline measurements from pristine environments are critical to the interpretation of other data).† Should an institution or foundation spend money studying Glacier Bay or say, oxygen depleted dead zones off their own coast which threaten local fisheries (as is the case in Oregon)? We, and the National Park Service hope that this cruise will spark some new interest in Glacier Bay research.† Even a location as pristine as this is not free from anthropogenic stress, and we have to establish a baseline now if we hope to understand these effects later. -Jeff Question: Domoic acid is consistently being found at high levels in razor clams on Washington coast resulting in digging closures. Is this something really new in the last few years or is it just easier to detect/test for these days? Do you expect to find higher or lower levels of domoic acid producing fauna in Glacier Bay as compared to open Alaskan waters or Washington coast? Why? Answer: Answer from April pending. Question: What makes sedimentation in Glacier Bay so different from sedimentation produced by a silty river dumping into a bay? Why does the lower bay have larger particle sizes (gravels?) than the upper bay? I would have thought that at the face of the glacier, larger rocks would drop out and not move too much and that fresh water inflow would sweep fine sediments away further into the bay before they settled out. Is there a distance from the face of the Glacier that sedimentation is so slight that other forces (tidal?) overcome the deposition rate and sweep the bottom surface clean? Answer: Only 250 years ago, Glacier Bay was almost completely covered by glacial ice.† Since then it has retreated nearly 90 km.† Glacier Bay is characterized by tall coastal mountains, temperate glaciers and the fjords that were carved out by these glaciers.†† This area is known for active tectonics, high uplift rates and massive glacial erosion. High basal lubrication of these temperate glaciers allows rapid glacial erosion to occur.† Sometimes these glacier termini directly feed into a fjord, which are then called tidewater glaciers.† This rapid erosion leads to some of the highest sediment accumulation rates in the world (Hallet et al. 1996).† These tidewater glaciers have been shown not to deposit sediment any further than five times the fjord width, for example Tarr Inlet ~ 2 km wide, sediment only accumulates down fjord ~ 10 km (Cai et al. 1997).
Scientists categorized three distinct zones in Tarr Inlet with in the tidewater glacier terminus proximity.†† Closest to the terminus is the Ice-proximal zone, characterized by morainal banks.† This area accumulates tens of cm/year to tens of m/year of unsorted sediments except for silts.† Further out from the terminus is the Ice-berg zone, characterized by laminated mud, weakly stratified diamicton, and stratified/massive sand and silt.† This zone accumulates sediments at rates of 100 to 19 cm/year respectively down fjord.†
Furthest away from the terminus is the Ice-distal zone characterized by interbedded homogenous/laminated mud, massive/stratified sand and coarse silt.† Sediment accumulation rates in this zone range from 6 to 3 cm/year down fjord respectively.† Previous studies from Cai et al. 1997 of showed that these tidewater glaciers can calve ice off of the glacier terminus, bringing with it an unsorted mix of rocks out into the bay.† Upon these icebergs melting, they deposit these rocks onto the fjord floor.† These icebergs can bring with them a variety of Ice Berg Rafted Debris (IBRD).† I think that finer sediments donít settle much further than the Ice-distal zone.† If you would like to know more about this, refer to Cai et al. 1997. Seismic Interpretation of temperate glacimarine sedimentation in Tarr Inlet Glacier Bay Alaska, Marine Geology 143: 5-37.† This paper truly inspired me and has some amazing figures and specs.† I hope at least answered part of your question.† Thank You.
-Justin Bergquist, University of Washington Question: The thin layers of phytoplankon and the associated concentration of first order predators (larvae) lead me to wonder about the concentration profile of higher level predators. Do they follow a similar concentration profile? Is the food web that tightly focused around a focal layer of only a few mm in breadth? Is the effect similar to a tide line in Puget Sound where debris, plankton, candlefish/herring and salmon all seem to be pushed/drawn together? Answer: Higher level predators follow this concentration profile in a way, but not exactly.† Where high concentrations of a prey (say herring as prey for salmon) exist you donít always find that their predators outnumber them with respect to location within the water column.† In fact, in most cases their predators certainly do not out number them, as herring swim in very large schools.† Iím arguing in this study that in these layers, predators (the larvae in this case) will be in higher concentration with respect to their prey at a specific location in the water column.† This is not applicable as you move up the food chain, to higher trophic levels, in most cases.
By tide line I assume you mean ďtidal frontĒ and no, this is not the same effect as a tidal front.† Thin layers form in areas where strong stratification exists, meaning the water is significantly layered with respect to differences in density.† At a frontal zone the water column is well mixed, meaning that layering is much weaker if density layers exist at all, and water is free to move vertically within the water column, typically moving deep, nutrient rich waters up to the surface.† It is this process of vertical mixing that promotes the growth of phytoplankton and draws candlefish/herring and salmon to these front in estuaries such as Puget Sound. -Jasper Question: Do you expect Glacier Bay to have less or more carbon take up than Puget Sound, the tropical Pacific or Lake Michigan? Why? What limits carbon take up in the oceans? I have read about the iron doping experiments to increase algae growth. Does this tie into the trace element study of iron that you are doing? On the other side of the carbon problem, what limits carbon from dropping out of the ocean? Is there a mechanism for increasing deposition rates on the ocean floor or will the seas just become increasingly more acidic? Answer: Thank you for your interest in my research!
Answering your first question about carbon uptake is complicated, because there are many parameters that must be considered to make estimates.† During the end of March, the Puget Sound should be the most productive of the basins you listed.† Both the Puget Sound and Glacier Bay are very productive, but I believe the Puget Sound is more due to its earlier bloom season and higher availability of nutrients.† I know very little about lake systems like Lake Michigan, but it is my understanding that freshwater basins are not typically as productive as saltwater areas of high productivity.
Here is a little background info that should be able to answer your questions about limiting factors in the ocean.† The sea surface takes up carbon via photosynthesis and carbon can also be taken up chemically if there is a lower concentration of CO2 in the sea surface water than there is in the surrounding air.† For photosynthesis to occur, phytoplankton (algae) needs nutrients that often limit their growth, and therefore their ability to uptake carbon.† Furthermore, the amount of available light (time of year) and temperature can trigger large blooms that cause a large amount of carbon to be uptaken.† As you can see, we are dealing with many different parameters to pinpoint the limiting factor.† In Glacier Bay on of the most common limiters of phytoplankton is light availability.†† There is plenty of nitrate and iron in the system, but often not enough light to get the photosynthesis occurring at full potential.† In the open ocean, at lower latitudes, the limiting factor is often iron.† My study isnít concentrating on Iron concentration, but I believe Marcus Petersonís is.† You can find his study on the ocean443 website at http://courses.washington.edu/ocean443.† Although, I can say that iron limitation is related to carbon uptake, because if plankton are limited by iron, their growth will be much slower and large amounts of carbon will not be able to be uptaken.
For your last questions, it sounds like you are wondering about how carbon uptake rates are related to carbon burial in sediment at the bottom of the ocean.† One of the primary concerns of the iron fertilization experiments is that sequestered carbon from the iron induced plankton blooms must actually make it to the sediment without being completely scavenged in the higher layers of the ocean.† This chemical and biological scavenging usually leads to increased respiration by organisms, which turns the carbon back into CO2.† Ocean acidity is directly connected with the amount of CO2 that the seawater is absorbing from the atmosphere.† If the concentration of the CO2 in the atmosphere and surface sea water is the same, no gas exchange takes place, but if there is a difference, CO2 will transfer to the reservoir with lowest concentration.† In most cases the sea water has a lower concentration and therefore acts as a sink for atmospheric carbon.† The ocean is getting more acidic due to the rise in atmospheric CO2 during the last 200 years.† This acidification will continue as long as the level of CO2 continues to rise Ė with little regard for vertical export of organic carbon.
-Andrew Clos Question: What levels of PAH do you expect to find in sediments? Are you expecting to see a profile that follows the expansion of the cruise ship industry into Glacier Bay? Will the Glacier Bay deposits show annual layers so that you can accurately make a historical log? How fast does PAH breakdown in a coldwater environment? Are there organisms that naturally breakdown PAH? Answer: Answer from Stefanie pending. Question: Is lignin so different from one tree species to another that you can tell them apart with a GC? What are the defining characteristics of a spruce lignin vs a hemlock lignin? For the lignins that have been washing into the bay, won't they be changed by their contact with sea water? Don't Cl and Na attack lignin, especially when oxygen levels are fairly high? Answer: Species can be identified through identification of lignin phenolic dimers. These dimers do show variation from species to species. Evergreens are particularly easy to identify. I will do identification at the grass-tree level using (LPVI) Lignin Phenol Vegetation Index, an index capable of sensitively detecting vegetation change and environmental changes that force those vegetation changes (Tareq, 2004). I am not well versed on the specific differences between tree species yet, so I canít say what the different between a spruce and a hemlock would be right now.
Chlorinated lignin can be created at pulp mills and maybe sewage treatment plants, but that process is not established in the Ocean. Super oxides (peroxide) are needed to create chlorinated lignin. If they are present, though, I will be able to measure them.
Degradation of lignin can occur converting them into Low Weight Molecules (LWM), but there are many published papers which display success in measuring this LWM lignin (Louchouarn, 2000). I will be able to measure lignin degradation by looking at the oxygenation of lignin in my samples.
Many thanks to Rick Keil for filling in the gaps and helping me understand all of this!
Louchouarn, P., S. Opsahl, R. Benner.. 2000. Isolation and Quantification of Dissolved Lignin from Natural Waters Using Solid-Phase Extraction and GC/MS. Anal. Chem. 72: 2780-7.
Tareq, S.M., N. Tanaka, K. Ohta. 2004. Biomarker signature in tropical wetland: lignin phenol vegetation index (LPVI) and its implications for reconstructing the paleoenvironment. Sci Total Environ. 324(1-3):91-103. Question: Is there an optimal amount of light scattering from sediment that would actually promote upper layer growth or does the light just bounce back to the atmosphere? Is there a trade off point between perfect clarity and few trace elements and higher turbidity and high trace element levels? Answer: The primary limiting factor for phytoplankton production is light, with nutrients such as iron being second.† Any light scattered forward (downward) into the water column can and will promote growth, as Glacier Bay is a fjord-like estuary with high sediment loads containing high amounts of nutrients.
Back scattering of light back into the atmosphere is a factor, but is considered ďsmall potatoesĒ once the light has penetrated into the water column.† Light is more likely to be strongly attenuated throughout the water column reducing the light available for production at regions in Glacier Bay where the sediment load and turbidity is high.† The more light that penetrates the water column the more can be used for carbon assimilation and increased productivity, but only to a certain extent.
Depending on the type of phytoplankton, some can show signs of photoinhibition, where the phytoplankton are over saturated with light, causing them to cease† photosynthesis and show signs of reduced production.† Some phytoplankton are very well shade adapted and can show signs of high productivity in areas heavily saturated in sediments with high turbidity.† So there is a balance to which light levels are optimal for phytoplankton photosynthesis and how much light is attenuated throughout the water column.
-Erwin Question: Could you describe a typical N isotope ratio profile from the surface down to the bottom of a body of Glacier Bay or some other body of water? How much does layer mixing disrupt the profile? In Glacier Bay, does fresh water intrusion from the glaciers result in salinity-defined N istope ratios? Answer: A typical N isotope ratio profile will have a positive increase with depth.† The phytoplankton at the surface are the primary producers and have the lowest N isotope ratio.† With each successive consumer level the isotope ratio increases.† The sediment at the seafloor would have the highest N isotope ratio.† In the surface water mixing will disrupt the measurement of isotope ratios mixing both primary producers and primary consumers, zooplankton, together.†
The impact of fresh water intrusion could have a large influence on the resulting N isotope ratios in the upper ~50m.† As you move further away from the glacier the impact decreases.† This influx of freshwater will impact the N isotope ratios in the surface layer depending on its effect on both phytoplankton and zooplankton.† Below the surface layer mixing will have a smaller impact on the resulting ratios and salinity is nearly constant.
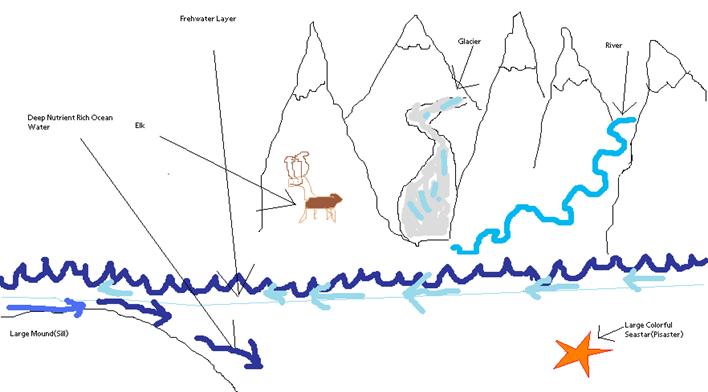
-Eric Question: Will the results from these studies published on this web site after the lab work and write ups are completed later this year? Answer: The tradition has been that each student's final paper is available on their personal website (go to the Ocean 444 link at left to access studentís sites), as well as in the University of Washington archives.† This year, since we have worked in close coordination with an outside agency (NPS) there are likely to be additional means of accessing this work, possibly as a single bundled file.† Stay tuned to this blog site and the Ocean 444 site, we will post that information later this spring. -Jeff Question: This question is regarding the project of Chase Stoudt, particularly his topic summary. Why does deep ocean water bring nutrient enrichment to the bay? From someone more than totally ignorant about all things oceanographic, I would just assume deep-sea water would carry fewer nutrients because of the lack of light and what I would guess to be a higher salinity. It seems like a far unfriendlier environment than the melted fresh water from glaciers, or even the surface ocean water that has more light exposure. In addition, why does knowing the origin of this deep ocean water matter?
Answer: Thatís a good fundamental question of oceanography, and forms the basis for a lot of studies involving estuaries like Glacier Bay. To answer this question we need to look at the structure of the ocean in general. Letís think up a hypothetical ocean which is very deep. All the things which live in the ocean die and sink to the bottom, where they decompose to nutrients that are used by phytoplankton (plants). The phytoplankton that lives in this ocean can only live near the surface where there is light, otherwise they will die. These phytoplankton then grow, reproduce, and photosynthesize using up all the available nutrients near the surface. Normally in the open ocean these nutrients are renewed by other processes (such as nitrogen fixation).
Now lets look at Glacier Bay which we can think of like our ocean with a few key differences. Glacier Bay has a very narrow opening compared with how large the bay is. This opening is further restricted by a large underwater mound (sill) at the mouth of the bay. We still have the phytoplankton growing and using up nutrients in the bay, but with no renewal of nutrients because of the constriction at the entrance. Thus the only way these phytoplankton can be fed is from ocean water making it into the bay.† This is why we need to know what kind of water is feeding the bay.† The special scenario with Glacier Bay is that we observe phytoplankton growing all the way through summer when they should have run out of nutrients.
As for the second part of your question, we need to examine the entrance and the areas surrounding Glacier Bay. As water exits the bay there are two ways it can go. One is to the open ocean and the other leads to Icy Strait and towards Juneau. These two areas are completely different; Icy Strait is a environment similar to Glacier Bay where the lack of nutrients limit phytoplankton growth. We donít know where the water which supplies Glacier Bay comes from (Ocean or Icy Strait) therefore my initial proposal was to track these waters. Unfortunately due to logistics I had to scrap this plan. I am now using surface drifters to calculate how much freshwater enters Glacier Bay from glaciers, rivers and other sources. This project is still relevant to† the initial question of how does deep ocean water bring nutrients to the bay. The freshwater which enters the bay can act as a barrier to this deep ocean water and can keep nutrient rich waters from making it into the bay. By measuring the amount of freshwater which enters the bay I can calculate how much of a barrier this water presents to nutrient rich deep ocean water. This has never been done in Glacier Bay before. A diagram of the discussed processes is below.
-Chase Stoudt
|


|
Quick Links
Senior Thesis Research Course, Ocean 444
Senior Thesis Planning Course, Ocean 443
Quick Links to Blog Entries
|
|
Glacier Bay banner background image by Andy Cameron, see original image. Send mail to: seniorcruise2008@ocean.washington.edu |