Methods and Materials
Conductivity Temperature Depth (CTD):
Field: A CTD is an instrument that is encased in a rectangular stainless steel cage that is lowered into the water using a cable powered by a small motor and guided by viable human hands. The model of CTD used was a Seabird 19. The instrument measures the conductivity-how much electric current passes through a water sample, temperature, depth, dissolved oxygen, fluorescence - this is a measurement of chlorophyll, which reflects the amount of phytoplankton in the water column, transmissivity, and calculates sigma-t (density). Samples were taken after the device was lowered to approximately 1 meter off the bottom, where a “messenger” - a solid brass weight- slid down the cable closes that closes the Niskin bottle. The device is then brought to the topside to collect the sample captured in the bottle.
Niskin bottle:
Field: A Niskin was used to collect bottom samples and was placed on the cable aproximately 1 meter above the CTD. The Niskin bottle works by keeping both ends open, so water can enter the bottle. The bottle is tripped as it rapidly closes and traps water for samples to be taken.
Niskin bottle on a Rosette:
Field: On larger boats, such as the Centennial, a CTD with a rosette will more than likely be used. Rosette's have several (from 12-36) Niskin bottles surrounding a CTD. Both the rosette and Niskin bottles have a stainless steel cable connected to the boat's computer program system in real time. Each bottle is electronically tripped at selected depths.
Scotty Bottle/Thoreson Bottle
Field: A Scotty bottle works similar to a Niskin bottle. It is used to capture water at a specific depth. The Scotty bottle is used to collect surface and thermocline water samples. Scotty works much the same way Niskin works except there is no copper messenger as a weight to send down the line. Instead, it is helpful to have extra hands on deck helping with this task to ensure the bottle is properly secured open before lowering the Scotty bottle over the side of the vessel. A quick jerking on the line will trip the bottle closed and trap the water samples taken at that depth. Great care must be taken to keep the bottle in the water and keep oxygen out of the sample so it does not skew the dissolved oxygen analysis.
Dissolved Oxygen (DO):
Field: Dissolved oxygen samples were collected from Niskin and Scotty bottles. This is done by attaching a small rubber tube to the spout of the bottle. The air bubbles need to be worked out while the glass Erlenmeyer flask is held upside down and rinsed. The flask is then held upright and filled as the tube and bottle are slowly being pulled away from each other as the water runs to the top of the flask and then the appropriate chemicals are added. Samples are taken back to the lab and processed using the adapted Winkler method (Carpenter 1965).
[Dissolved oxygen lab procedures]
Chlorophyll:
Field: Two samples were taken at each station using a 145ml numbered plastic bottle. Each bottle is rinsed with the collected sample three times. Bottles are filled past the top, sealed and then stored in a dark cooler on ice, as this prevents growth, since chlorophyll indirectly measures the amount of photosynthetic organisms that are in the water. Each sample is analyzed the same day it was collected to ensure accurate data is recorded for that snapshot in time of the productivity.
[Chlorphyll lab procedures]
Nutrients:
Field: Sample water from both Niskin and Scotty bottles were put in a syringe with a 25 micro filter attached. The filtered water is used to rinse out the syringe and nutrient bottle three times. After the rinse is complete, the bottle is filled two thirds full with filtered water and put in a cooler on ice for preservation. The samples are then frozen and sent off to UW Seattle to be analyzed. One nutrient sample was taken at the net, surface, and thermocline depths.
Secchi:
Field: A secchi is a round type of disc that is black and white in color and attached to a double braided line to toss over the side of the boat to measure the penetration of sunlight through the water column. The same person lowers the disk into the water for the entire trip to ensure the measurements remain consistent and accurate. The disc was lowered until it could no longer be seen and the depth was recorded where it was last visually seen by the meter marks on the line. The angle of the line was also taken into consideration for currents and measurements were taken on the shady side of the boat.
Phytoplankton
Field: Plankton samples were collected at each station using a 20 micron net. Sampling is done by pulling the net horizontally at the surface level. The water is poured into a pre-labeled glass sample jar. Each sample from the net tow were prepared and counted under a microscope at the University of Washington Tacoma laboratory. A pre-labeled glass jar is filled from both the
both Niskin and Scotty bottles, depending on the depth samples were taken. The jars are then put in a dark cooler on ice to prevent population growth and ensure accurate data is recorded at the time the sample was taken from the selected station. Phytoplankton were observed and counted using techniques in Horner [2002].
[Phytoplankton lab procedures]
Zooplankton catch net:
Field: The Zooplankton net is 153 micrometers and descends to a predetermined depth and then is retrieved 50 meters at which point a messenger is sent to trigger the closure of the net. This ensures that only specific depth ranges are sampled.
Van Veen/Ponar:
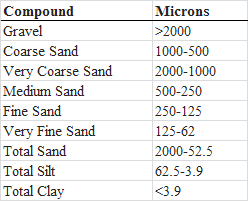
Field: This equipment is used after all the samples at the station are completed, since this sample is to collect sediment. It has a spring loaded pin that triggers the grab when it touches the bottom. This method is repeated until sediments are collected into the device. After sediments are retrieved they are transferred to a labeled plastic baggie, and then placed on ice in a dark cooler and taken back to the lab and sorted for grain size by using the Coulter LS 200 Particle Size Analyzer. The total organic content (TOC) is calculated in the laboratory by loss on ignition.
[Sediment particle size analysis]
[Total organic content]
Microplastics:
Field: This method requires a specialized Manta net specifically designed and calibrated to sample the top ~ 20 centimeters of surface water. It is towed behind a boat and used to collect plastic debris. Plastics include hard, soft, styrofoam, filaments, foams, fragments, and pellets.
[Microplactic lab procedure]
Methods and Materials
References
- Carpenter, J.H. 1965a. The accuracy of the Winkler method for dissolved oxygen. Limnology and Oceanography 10: 135-140.
- Strickland, J.D.H.; Parsons, T.R. 1972. A Practical Handbook of Seawater Analysis. The Alger Press Ltd. Ottowa.
- Horner, R. 2002. A Taxonomic Guide to Some Common Marine Phytoplankton, Biopress Ltd., Bristol, England.
Created by Kyra Gagliardi, Carolyn Green, and Jonathan Silas