
A Primer on Arterial Blood Gas Analysis
by Andrew M. Luks, MD
Print primer [237k PDF*]
- Introduction
- Acid-Base Status
- Terms and Normal Values
- How the Data Are Presented
- Before you get started
- A Step-wise Approach to Interpretation
- Step 1: Examine the pH and compare it to the normal range
- Step 2: Determine the primary process that led to the change in the pH
- Step 3: Calculate the serum anion gap (SAG)
- Step 4: Identify the compensatory process (if one is present)
- Step 5: Determine if a Mixed Acid-Base Disorder is Present
- Generating Differential Diagnoses
- Elevated Anion Gap Metabolic Acidosis
- Normal Anion Gap Metabolic Acidosis (non-gap acidosis)
- Metabolic Alkalosis
- Respiratory Acidosis
- Respiratory Alkalosis
- Use Old Arterial Blood Gas Results to Your Advantage
- Oxygenation
- Assessing the Cause of Hypoxemia
- Assessing the Adequacy of Gas Exchange
- The P/F Ratio
- The AaO2 Difference
- Special Situations To Be Aware of In Evaluating the PaO2 on an ABG
Introduction
Arterial blood gases play an important role in the work-up and management of critically ill patients and patients with a variety of pulmonary complaints and disorders. For example, they are used to guide the adjustment of ventilator parameters on mechanically ventilated patients and are also a standard part of the work-up of patients who present with unexplained hypoxemia or dyspnea. It is, therefore, important that students and physicians be able to interpret the results of arterial blood gas sampling, determine the patient's acid-base status and assess the adequacy of oxygenation.
This primer describes a clinical approach to interpreting arterial blood gases. It will outline a step-wise approach to interpreting the acid-base status and generating differential diagnoses for the observed problems. It will then address the proper means for assessing the adequacy of oxygenation and determining the etiology of any observed abnormalities.
The physiologic principles underlying acid-base physiology are beyond the scope of this review and will not be considered here, but information about this topic can be obtained from the following additional resources:
- HuBio 541 Course Syllabus:
http://courses.washington.edu/hubio541/secure/syllabus/06AcidBase.pdf [1.5M pdf*] - Murray and Nadel's Textbook of Respiratory Medicine: Chapter 7 – Acid-Base Balance
http://www.mdconsult.com/das/book/body/139612442-2/0/1288/55.html?tocnode=51470230&fromURL=55.html#4-u1.0-B0-7216-0327-0..50010-0_294
Acid-Base Status
Terms and Normal Values
Before reviewing the assessment of acid-base status, it is helpful to review the normal values for the main acid-base parameters and some basic terminology.
The normal values for acid-base parameters are as follows:
- pH: 7.38 - 7.42
- PaCO2: 36 - 44 mmHg
- Bicarbonate: 22 – 26 mmol/L
Be aware that the normal ranges for these parameters will vary slightly from laboratory to laboratory.
The following terminology is applied to acid-base interpretation:
- Acidemia: refers to a low blood pH (< 7.38). Patients with a low pH, are said to be "acidemic."
- Alkalemia: refers to a high blood pH (> 7.42). Patients with a high pH are said to be "alkalemic."
- Acidosis: refers to any process that, if left unchecked, will lead to acidemia. This can occur through one of two mechanisms.
- A respiratory acidosis is present when the PCO2 is high (> 44)
- A metabolic acidosis is present when the HCO3- is low (< 22)
- Alkalosis: refers to any process that if left unchecked will lead to alkalemia. This can occur through one of two mechanisms.
- A respiratory alkalosis is present when the PCO2 is low (< 36
- A metabolic alkalosis is present when the HCO3- is high (> 26)
It is important to keep these terms straight in your mind and in your communications with others. It is common for people to refer to the patient with a low pH, for example, and say they are "acidotic." Similarly, they often refer to the patient with a high pH as "alkalotic." This is incorrect terminology.
When you are referring to the patient and their pH, the correct terminology is as follows:
- The patient with a low pH has "acidemia" or is "acidemic."
- The patient with a high pH has "alkalemia" or is "alkalemic."
How the Data Are Presented
While the laboratory will always label each value in the arterial blood gas results, it is not uncommon for residents, fellows and attending physicians to either write or state the results without labeling each value. For example, rather than stating: "the pH is 7.40, the PCO2 is 40, the PO2 is 85 and the HCO3- is 24" they may simply state or write: "7.4/40/85/24."
If ABG results are presented in this manner, by convention, they will be written or spoken in the following order:
pH ![]() PCO2
PCO2 ![]() PO2
PO2 ![]() HCO3-
HCO3-
Before you get started…. Make Sure the Numbers Are Consistent
Before you do you acid-base interpretation, it is important to do a little troubleshooting and make sure there are no measurement errors with your blood gas results.
There are two things you should do.
First, make sure it is an arterial sample and not a venous sample. The best way to do this is to observe how the blood comes back into the blood gas syringe as the sample is drawn. Pulsatile flow is seen with an arterial sample but would be lacking with a venous sample. Similarly, arterial samples usually fill the syringe quickly, while venous samples move much more slowly into the syringe. You cannot always rely on the color of the blood to tell you it is arterial because a very hypoxemic patient will have dark, "venous-appearing" blood. If you did not see the sample as it was drawn into the syringe, you can use the PO2 as a guide. If the patient was not very hypoxemic when the blood gas was drawn but you get a very low PO2 with the results (30s-40s), it is likely that you have a venous sample. This tactic is a bit harder to use when the patient is very hypoxemic when the sample is drawn.
Second, you should make sure there are no measurement errors. A simple way to do this is to compare the bicarbonate value from the blood gas (a calculated value) with the bicarbonate from the chemistry panel (a measured value). They are not always exactly the same but they should be close to each other. This only works, however, if your chemistry panel and blood gas were measured at roughly the same time. You cannot do this if the samples were drawn many hours apart.
A more thorough approach is to see if there is consistency between the blood gas and the chemistry panel using the Henderson-Hasselbach equation. The equation is used to calculate the pH you would expect based on the measured PCO2 and HCO3-. This pH is then compared to the measured pH. If the values are similar, your sample is valid. If the values are far apart, there may be a measurement error.
Because no one can easily remember the full Henderson-Hasselbach equation, there is a modified process that can be used instead. This modified process is as follows:
- Calculate the hydrogen ion concentration using a modified Henderson-Hasselbach equation: [H+] = 24 x PCO2 / HCO3-
- Use the calculated [H+] to determine what the pH should be. pH is a function of the hydrogen ion concentration (pH = -log [H+]) but rather than doing the calculation you can refer to the following table:
If the [H+] is… Then the Calculated pH is… 100 7.00 79 7.10 63 7.20 50 7.30 45 7.35 42 7.38 41 7.39 40 7.40 39 7.41 38 7.42 35 7.45 32 7.50 25 7.60
- Compare the calculated pH to the measured pH. If they are similar, your sample is valid. If the values are far apart, there may be a measurement error.
A Step-Wise Approach to Acid-Base Status Interpretation
As with reading an electrocardiogram or a chest x-ray, it is important to use a system when reading arterial blood gases. Adhering to a system will allow you to identify the primary and compensatory process and any additional disorders that may be present.
A suggested step-wise approach for reading an arterial blood gas is as follows:
- Examine the pH and comparing it to the normal range
- Identify the primary process that led to the change in pH
- Calculate the serum anion gap
- Identify the compensatory process (if one is present)
- Identify if any other disorders are present or there is a mixed acid-base process
Each of these steps is described below in greater detail. After working through these steps, you should be able to give a one- or two-sentence synopsis of the patient’s acid-base status such as "This patient has a primary respiratory acidosis with a compensatory metabolic alkalosis."
As you go through this process, try not to lose track of the clinical scenario that led to the blood gas being drawn in the first place. You will use the results and their interpretation to help you figure out what is going on with the patient. In addition, you should always ask if the results make sense in light of what you know about the patient’s case. If the results do not make sense, either your interpretation was wrong or there may be some additional processes at work that were not recognized on the initial analysis.
With that in mind, the main steps in interpreting an arterial blood gas in greater detail are presented below.
Step 1: Examine the pH and compare it to the normal range.
As noted above, if the pH is low, the patient has an acidemia. If the pH is above this range, the patient has an alkalemia. Be aware that patients can have mixed metabolic disorders (e.g., concurrent metabolic acidosis and alkalosis) that can give them a pH in the normal range. This will be discussed further below.
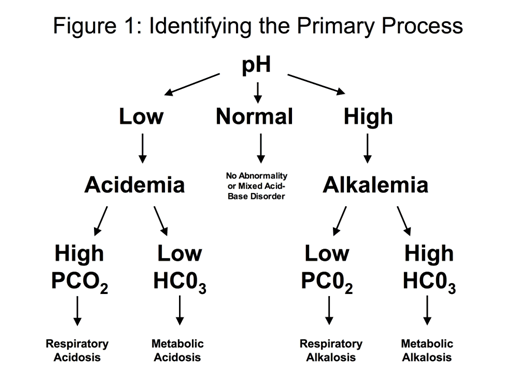
Step 2: Determine the primary process that led to the change in the pH:
For a patient with a low pH (acidemia)
- If the PCO2 is elevated (> 44), the primary process is a respiratory acidosis
- If the HCO3- is low (< 22), the primary process is a metabolic acidosis
For a patient with a high pH (alkalemia)
- If the PCO2 is low (< 36), the primary process is a respiratory alkalosis
- If the HCO3- is high (> 26), the primary process is a metabolic alkalosis.
This framework is depicted in Figure 1.
Some examples of how to work through this first step in the process›
Step 3: Calculate the serum anion gap (SAG)
Serum Anion Gap (SAG) = Na+ - (Cl- + HCO3- )
You should use the bicarbonate from the chemistry panel for this calculation. If this value is elevated (> 12), the person is deemed to have an "elevated anion gap." This implies that the patient has a primary elevated serum anion gap metabolic acidosis regardless of what other abnormalities you identify or what else is happening with the pH and bicarbonate. There must be an additional disorder because the body does not generate an anion gap in order to compensate for a primary respiratory disorder.
Be aware, however, that an elevated anion gap acidosis may not be the only primary process. For example, patients with salicylate intoxication may have a primary respiratory alkalosis and a concurrent primary elevated anion gap acidosis at the same time.
An example of how to work through this situation›
You should also note that the normal anion gap is affected by the patient's serum albumin level. As a general rule of thumb, the normal anion gap is roughly three times the albumin value. By way of example, for a patient with an albumin of 4.0, the normal anion gap would be 12. For a patient with chronic liver disease and an albumin of 2.0, the upper limit of normal for the anion gap would be 6. Other people propose that the ceiling value for a normal anion gap is reduced by 2.5 for every 1g/dL reduction in the plasma albumin concentration.
Page 1 | go to Page 2
*Software capable of displaying a PDF is required for viewing or printing. You may download a free copy of Adobe Reader from the Adobe website.
UW School of Medicine : School of Medicine Mission
Copyright and Disclaimer : Credits and Acknowledgements