
A Primer on Mechanical Ventilation
by David J Pierson MD (cont.)
back to Page 1 | Page 2
Guidelines for Ventilator Settings in Different Clinical Circumstances
Table 8 [43k PDF*] summarizes the recommended approach to setting the ventilator depending on the patient’s acute respiratory problem and the clinical setting.
Routine ventilatory support
Most patients who require a period of invasive mechanical ventilation have relatively normal underlying lung function. What may be referred to as “routine ventilatory support” is encountered most frequently in the postoperative period or in the setting of short-term loss of spontaneous ventilation, such as with a drug overdose. In such settings, a volume-targeted mode such as A/C ventilation is simplest and most reliable, and usually requires fewer adjustments than does pressure-targeted ventilation. Some clinicians prefer to add 5 cm H2O of PEEP routinely in order to counteract the modest drop in functional residual capacity (FRC) that has been shown to occur with endotracheal intubation, although this is probably unnecessary in most patients.
Healthy individuals normally breathe with a Vt of 5-7mL/kg. However, diffuse microatelectasis and an increased difference between alveolar and arterial oxygen tensions [P(A-a)O2] soon develops because of decreased surfactant function if these individuals do not more fully expand their lungs several times per hour by sighing. The same problem exists with intubated patients who have normal lungs and who are ventilated with ‘normal’ Vts in the absence of sigh breaths. The need for sighs, which in the presence of pulmonary disease might overdistend and rupture alveoli, can be obviated if a larger Vt (10-12 mL/kg) is used.
The cycling rate and hence minute ventilation are adjusted to provide normal arterial pH and PaCO2 values (e.g., 7.40 ± 0.05 units and 40 ± 5 mmHg, respectively). Enough supplemental oxygen is used to prevent hypoxemia, although maintaining PaCO2> 100 mm Hg is unnecessary.
Obstructive lung disease
Compared to individuals who do not have chronic lung disease, patients who have severe COPD or asthma are at increased risk for circulatory impairment and barotrauma when subjected to invasive mechanical ventilation, and a number of modifications of the routine approach are required to avoid these.
The most common problem is pulmonary hyperinflation, which patients who suffer obstructive lung disease typically have at baseline, and which worsens during times of acute exacerbation. The three main goals of invasive mechanical ventilation in patients who have acutely exacerbated COPD or acute severe asthma are to:
- rest the ventilatory muscles;
- avoid further dynamic hyperinflation;
- avoid overventilation and acute alkalemia.
Resting the ventilatory muscles may be achieved either by providing full ventilatory support using volume-targeted ventilation (with either A/C ventilation or SIMV), so that the patient makes no respiratory effort, or by providing partial ventilatory support using PSV, such that tachypnea and respiratory distress are relieved. If the former strategy is used, Vts of 5-7 mL/kg and a rapid inspiratory flow (e.g., 80–100 L/min) to maximize expiratory time and avoid air trapping (see subsequent section on auto-PEEP) are recommended.
This is one of the two clinical settings in which permissive hypercapnia is appropriate, the other being acute lung injury as discussed below. When the degree of airflow limitation is severe, it may not be possible to provide a sufficient minute ventilation (i.e., respiratory rate x Vt) to reduce the PaCO2 enough to produce a normal pH without a resultant worsening in hyperinflation. In obstructive lung disease, PEEP serves a different function from that in acute lung injury. Its purpose here is not to increase lung volume (which is already excessive), but to decrease the muscular effort required to trigger the ventilator or breathe spontaneously in the presence of dynamic hyperinflation and auto-PEEP.
Acute Lung Injury and the Acute Respiratory Distress Syndrome
The goals of mechanical ventilation in acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are to:
- support oxygenation;
- avoid circulatory compromise; and
- avoid ventilator-induced lung injury.
The first of these goals is accomplished through manipulations of FIO2 and PEEP, the aim of which is to balance the risks of pulmonary oxygen toxicity with those of raised intrathoracic pressures and lung volumes. Attempts to avoid ventilator-induced lung injury include a ‘lung-protective’ ventilation strategy that involves low Vt, limited alveolar pressure, and permissive hypercapnia.
Either volume- or pressure-targeted ventilation may be used to manage ALI-ARDS. The use of PCV has several potential advantages, although whether these lead to improved outcomes or fewer complications remains to be determined. Whatever mode is used, it has been demonstrated both in animal models and in patients that the larger-than-physiologic Vts used for routine ventilatory support contribute to lung injury. Current best evidence indicates that a "lung-protective" ventilation strategy that keeps VT to a maximum of 6 mL/kg predicted body weight and avoids end-inspiratory plateau (static) pressures above 30 cm H2O minimizes ventilator-induced lung injury and reduces mortality.
Because of the increasing prevalence of obesity in the developed world, and the risk of unintended lung overdistention in overweight patients if admission weight rather than predicted body weight is used to determine VT, the following formulas are used to determine initial settings:
Males
Predicted body weight (kg) = 50 + 2.3 [(height in cm - 152) divided by 2.54]
= 50 + 2.3 (height in inches - 60)
Females
Predicted body weight (kg) = 45.5 + 2.3 [(height in cm - 152) divided by 2.54]
= 45.5 + 2.3 (height in inches - 60)
The rate must be adjusted so that auto-PEEP does not develop, a limitation that results in hypercapnia in many patients. Most patients tolerate arterial pH values in the range 7.20-7.30 without difficulty, and bicarbonate infusion is not used by most authorities unless the value falls well below this range.
In managing patients who have severe ARDS, the clinician may need to accept ‘permissive hypoxemia’ as well as permissive hypercapnia. Although the goal is to maintain PaCO2 in the normal range, this may not be attainable in some patients without the use of potentially injurious levels of PEEP. A PaCO2 of 50-60 mm Hg [oxygen saturation as measured by pulse oximetry (Spo2) 80–90%] is usually well tolerated if hemoglobin concentration and cardiac function are adequate. As both hypercapnia and hypoxemia distress patients, appropriate sedation is required when pursuing the ‘lung protective’ ventilatory strategy in ARDS. The "PEEP-FIO2 Ladder" (Table 9 [20k PDF*]) titrates these two ventilator settings according to the original ARDS Network protocol. A second study comparing this "ladder" for setting PEEP and FIO2 to a similar titration employing higher PEEP levels and correspondingly lower FIO2s found no differences in patient outcomes.
Unilateral or Markedly Asymmetric Lung Disease
Patients who have lobar pneumonia, lobar or whole-lung atelectasis, and other markedly asymmetric pulmonary involvement present a special problem in mechanical ventilation, particularly in the presence of severe hypoxemia. Such patients illustrate why PEEP should not automatically be applied as treatment for hypoxemic respiratory failure. Respiratory system compliance is much higher in relatively normal areas of lung than in areas of consolidation or collapse. As a result, application of PEEP may preferentially expand these more normal areas and not produce the desired effect in the involved lung. Distention of normal lung tissue stretches and narrows pulmonary vessels, which can raise pulmonary vascular resistance sufficiently to divert blood to the abnormal areas. Accordingly, applying PEEP can worsen rather than improve arterial oxygenation in such instances. Some patients who have hypoxemic respiratory failure and apparently asymmetric lung involvement respond favorably to PEEP, however, which emphasizes the need to perform PEEP trials as routine.
Neuromuscular disease
Patients who have acute neuromuscular disease or cervical spinal cord injury and whose lung function is relatively normal may benefit from ventilator management at higher than usual Vts and flows. Such patients may experience dyspnea at Vts of 10-12 mL/kg, which improves when larger volumes (12-16 mL/kg) are used, although this is controversial. Similarly, these patients typically prefer faster inspiratory flows (e.g., 80–100 L/min). Such settings often result in a mild-to-moderate respiratory alkalosis, which is usually well tolerated and soon accompanied by a compensatory metabolic acidosis. Alternatively, low levels of PEEP may accomplish the same relief of dyspnea as the attendant hyperventilation.
Many patients who have traumatic quadriplegia or other acute neuromuscular disorder experience recurrent atelectasis, which can cause more severe hypoxemia than is usually seen with lobar collapse in other clinical settings. Ventilation with larger than usual Vts, with or without the addition of low-level PEEP, is important in such patients to prevent recurrence. Frequent changes in posture may also be beneficial.
Acute brain injury
Patients who have closed head injury or other acute brain insult may lose the normal autoregulation of cerebral perfusion pressure (CPP). In such patients anything that decreases mean arterial pressure or raises central venous pressure must be avoided. Thus, PEEP is used cautiously, if at all, in patients who have acute brain injury, as the raised intrathoracic pressure is transmitted via the vertebral veins to the central nervous system. Maneuvers that induce coughing and may raise intracranial pressure, such as tracheal suctioning, are avoided whenever possible in these patients.
For many years, deliberate hyperventilation to arterial PaCO2 levels of 25-30 mmHg was an integral part of ventilator management in patients who had acute brain injury. Results of recent studies call this practice into question, however, and hyperventilation is no longer used in many institutions, except as a temporary emergency measure while other treatments for intracranial hypertension are initiated.
Flail chest
Several studies demonstrate that the clinical course and outcome of flail chest injury are determined mainly by the underlying pulmonary injury rather than the flail segment per se. Patients who sustain multiple rib fractures without associated lung contusion or pneumonia generally recover uneventfully, while flail chest in the setting of acute lung injury typically follows the course of that illness, with little separate contribution from the chest wall instability.
Ventilator management of patients who have a flail chest injury is thus essentially that used to manage the underlying pulmonary condition. Attention must be given to pain control, however, particularly when ventilator modes providing only partial ventilatory support are used, as these force the patient to use the intercostal muscles associated with the flail. Many clinicians prefer to use full ventilatory support until the patient is ready for weaning. Intercostal nerve blocks or administration of epidural narcotics can greatly aid in pain control and ventilator weaning in such patients.
Weaning and Extubation
The clinician faces multiplicity of options when reducing or discontinuing ventilatory support, which has resulted in considerable controversy regarding the ‘best’ approach. In the last decade there has been a fundamental change in the approach to discontinuing invasive mechanical ventilation--from "predicting" to "checking."
For the last 30 years of the Twentieth Century clinicians used a variety of measures of ventilatory mechanics in attempting to identify the point at which a patient was ready to switch from full ventilatory support to fully spontaneous breathing. These predictive measures or "weaning parameters" included spontaneous respiratory rate, often divided by the patient's average spontaneous tidal volume during a brief disconnection from the ventilator (f/VT, or the "rapid shallow breathing index"), inspiratory vital capacity, minute ventilation, and the maximum inspiratory pressure the patient could generate during end-expiratory airway occlusion. Most often, patients remained fully supported until threshold criteria were reached in one or more of these "weaning parameters," and then a trial of spontaneous breathing or progressively reduced inspiratory support was undertaken.
A systematic review of existing evidence for the efficacy of the substantial number of weaning predictors, singly and in combination, was undertaken in conjunction with the development of international practice guidelines for weaning [ref-2]. Two important findings of this process were that no existing measurement, index, or combination of measurements could predict the ability to discontinue ventilatory support with sufficient accuracy, and that many patients considered by the clinicians managing them to be "unweanable" or in need of a prolonged weaning regimen could, in fact, breathe fine without assistance if simply taken off ventilatory support. This led to the recommendation that, rather than attempting to predict when patients were ready for weaning, clinicians should simply "check," both early and repeatedly, to determine whether they were, in fact, ready.
Assessment for Readiness to Wean
According to the new evidence-based guidelines, patients should be checked for readiness to wean when the following conditions are present:
- Evidence for some reversal of the underlying cause for acute respiratory failure;
- Adequate arterial oxygenation (for example, arterial PO2 at least 60 mm Hg on 40% oxygen with positive end-expiratory pressure 5 cm H2O or less);
- Acceptable acid-base balance (for example, arterial pH 7.25 or higher);
- Hemodynamic stability (absence of active myocardial ischemia, and blood pressure supportable without requirement for significant vasopressor support); and,
- Sufficient ventilatory drive and neuromuscular function to initiate a spontaneous inspiratory effort.
The Spontaneous Breathing Trial
Patients meeting the above criteria should have a spontaneous breathing trial. Although the time-honored T-piece method remains the favorite of many clinicians, this requires disconnecting the patient from the ventilator and interposing a different breathing circuit. A simpler alternative is to leave the patient connected to the ventilator circuit and switch to the CPAP mode, so that no mandatory or assisted breaths are provided. Low-level CPAP (e.g., 5 cm H2O) can be provided during the trial at the discretion of the clinician, although adding more than 5 cm H2O of inspiratory pressure support tends to defeat the purpose of the spontaneous breathing trial and should be avoided. Increasing the FIO2 by 0.10 during the trial is recommended to avoid hypoxemia, as lung volumes and ventilation-perfusion matching may change during spontaneous breathing.
Patients should be observed closely during the trial for evidence of increasing respiratory distress (such as progressively increasing respiratory rate over several minutes to 35 breaths/min or more), or for increasing tachycardia or systemic arterial hypertension, indicating that ventilatory support should be reinstituted. If spontaneous breathing is clinically tolerated for 30 minutes, an arterial blood specimen should be obtained for analysis to confirm that arterial oxygenation and acid-base status have not deteriorated unacceptably. The trial may be extended for up to 120 minutes if it is not initially clear whether it will be successful. However, there is little to gain by lengthening the period of spontaneous breathing beyond this point,. Prompt extubation should be considered for patients who tolerate the trial clinically and maintain acceptable arterial blood gas values, providing no separate indication for an endotracheal tube is present (see below).
Evaluation of the Patient Who Fails a Spontaneous Breathing Trial
An unsuccessful spontaneous breathing trial in a patient who fulfills the criteria for initiating it should be regarded as a diagnostic and therapeutic problem requiring deliberate attention. Table 10 [58k PDF*] presents an algorithm for sorting out the reason or reasons for a failed trial. According to best available evidence, patients should undergo a spontaneous breathing trial once each day. Between trials, a level of support should be provided (such as assist-control ventilation, or pressure support sufficient to prevent tachypnia and maintain patient comfort) such that the patient is able to rest without development of ventilatory muscle fatigue.
For difficult cases, as with prolonged mechanical ventilation in acute respiratory failure or in patients who have a serious underlying illness in other organ systems, it is helpful to approach weaning in a systematic fashion, considering possible impediments to success in approximate order of their likelihood, as summarized in Table 9. In most cases of initial weaning failure, the reason becomes apparent on proceeding through the algorithm in a stepwise fashion. Indeed, perhaps the most common reason for inability to wean from mechanical ventilation among patients who have been critically ill for a week or more is that the primary illness has not improved sufficiently for weaning to be achievable.
When patients are unable to be weaned because of a high minute ventilation requirement, as is commonly seen in ARDS, the clinician may be helped by an analysis of the physiologic mechanism responsible. Only three basic mechanisms can account for a higher than normal minute ventilation: hyperventilation (i.e. respiratory alkalosis), increased carbon dioxide production, and increased dead space ventilation (Vd/Vt). Hyperventilation is readily identified by the presence of hypocapnia. Carbon dioxide production and Vd/Vt can be assessed either by collecting expired gas in a bag or using a metabolic cart (as used for determining nutritional requirements), in conjunction with an arterial blood sample. Making these simple measurements can aid in identifying the reason for a high minute ventilation:
- If increased carbon dioxide production is the cause, the clinical problem is a systemic rather than pulmonary one—perhaps the patient is receiving excessive nutritional support;
- If increased Vd/Vt is responsible, increased ventilation requirement results from inefficient ventilation, as seen with ARDS, dynamic hyperinflation, or pulmonary thromboembolism.
Weaning technique
The main techniques currently in use for gradual weaning from ventilatory support are the T-piece method and PSV. The time-honored T-piece method consists of repetitive periods of spontaneous ventilation to the patient’s tolerance, interspersed with periods of rest with full ventilatory support. When PSV is used in weaning, the patient is switched from full ventilatory support to PSV at an inspiratory pressure sufficient to provide the same Vt as before; inspiratory pressure is then gradually reduced, using the patient’s respiratory rate (usually maintained below 30 breaths/min) as a guide to the adequacy of support. Weaning with SIMV, which consists of gradually reducing the mandatory rate and thus progressively decreasing the ventilator’s contribution to the required minute ventilation, using the total respiratory rate (mandatory plus spontaneous breaths) and other clinical signs as indicators of progress, is no longer recommended because multicenter clinical trials have shown it to prolong the process when compared to T-piece trials and PSV.
Extubation
Discontinuation of ventilatory support and extubation are not the same thing. In most instances, both can be carried out together – as after general anesthetic. However, failure to recognize the important exceptions can lead to serious problems. In addition to the need for mechanical ventilation, indications for translaryngeal intubation include upper airway problems, inability to protect the lower airways from aspiration of oropharyngeal or gastric contents, and inability to clear secretions without suctioning or other measures.
Unfortunately, no specific tests or measurements are currently available to assess patients’ ability to protect their airway from aspiration and their ability to clear secretions. A trial of extubation is often the best way to determine whether the patient needs continued airway protection (with a tracheostomy, for example).
Complications Associated With Invasive Mechanical Ventilation
Although frequently life saving, invasive mechanical ventilation is also associated with numerous complications, some of which can be life threatening in themselves (Table 11 [24k PDF*]). The expression ‘associated with’ is important conceptually, as many adverse effects that occur while a patient is being mechanically ventilated cannot be shown conclusively to arise directly from the ventilator or its operation. Nosocomial pneumonia in the ventilated patient, for example, is more likely a consequence of critical illness and the breakdown of normal host defenses than of the ventilator per se. Since they are related temporally and clinically to intubation and mechanical ventilation, however, it is logical to consider all such complications together here.
Physiologic effects of positive-pressure ventilation
Initiating positive-pressure mechanical ventilation in any patient alters pulmonary mechanics and respiratory function. Several of these changes are more ‘effects’ than ‘complications’ in that they are direct, predictable results of changes in lung volume and intrathoracic pressure. In several instances, although these effects have been well known for years, the precise mechanisms remain uncertain.
Impaired cardiac function
The effects of positive-pressure ventilation on cardiac function are more common with the application of PEEP, but may also occur when mechanical ventilation is used without PEEP, especially in volume-depleted patients and those who have pre-existing cardiac disease.
Several mechanisms for decreased cardiac function have been postulated, and which is most important remains unclear. However, most investigators agree that two mechanisms predominate:
- reduced right ventricular preload as a result of raised mean intrathoracic pressure (which is an absolute effect of positive pressure ventilation); and
- increased right ventricular afterload because of increased lung volume (which need not occur if gas trapping does not occur and if tidal residual volumes are physiologic).
Compared with spontaneous breathing, positive-pressure ventilation increases both peak and mean intrathoracic pressure, and thus increases the pressure gradient that must be overcome by venous blood as it returns to the right atrium. Mean intrathoracic pressure is further increased by application of PEEP, and in the presence of relative hypovolemia, even small amounts of PEEP can compromise cardiac function. Patients who have pre-existing right ventricular dysfunction are at increased risk for cardiac impairment on addition of PEEP. Some investigators have suggested that the reduction in venous returns may be the result of an increase in inferior vena caval resistance because of the increase in lung volume rather than the decrease in driving pressure grouping venous return.
Marked increases in lung volume cause an increase in pulmonary vascular resistance. This increases right ventricular afterload, which may compromise the forward output of that chamber. The increase in right ventricular volume associated with raised pulmonary vascular resistance may in turn impair left ventricular function, since both ventricles share the interventricular septum, pericardial sac, and certain circumferential muscle fibers.
Positive-pressure ventilation does not always impair cardiac function, and in certain circumstances it may even improve it. Although the work of spontaneous breathing normally accounts for a small proportion of overall oxygen consumption, this may increase drastically during acute respiratory failure. When cardiac function is severely impaired, as in cardiogenic shock, the heart may not be able to meet the demands imposed by this excessive work of breathing. In addition, the increase in juxtacardiac pressure that occurs as a result of the increase in intrathoracic pressure induced by positive pressure causes a functional reduction in left ventricular afterload.
Increased intracranial pressure
Positive pressure ventilation can increase jugular venous pressure, which in turn can impede venous return from the brain and raise increased intracranial pressure (ICP).
Normally, over a wide range of CPP (i.e. mean arterial pressure minus ICP), cerebral blood flow is kept constant through autoregulation, and changes in ICP or cardiac output have little effect. When ICP is elevated, however, as after head injury, autoregulation is lost, the relationship of cerebral blood flow to CPP becomes linear, and either an increase in ICP or a drop in cardiac output can reduce cerebral blood flow. Fortunately, conditions such as ARDS that require high levels of PEEP to support oxygenation also markedly reduce lung compliance, so that less pressure is transmitted to the jugular venous system.
Gastric distention
Gastric and intestinal distention with air may occur when manual ventilation with bag-and-mask raises mouth pressure above lower esophageal sphincter pressure. During mechanical ventilation via a cuffed endotracheal tube, it is also not uncommon for patients who have low respiratory system compliance to develop gastric distention, presumably because tracheal pressure exceeds both cuff pressure and lower esophageal sphincter pressure in the presence of a closed or occluded mouth. Distention can be massive (‘meteorism’), and gastric rupture has been reported. Placement of a small-bore nasogastric tube usually prevents or alleviates this problem.
Respiratory alkalosis
Respiratory alkalosis is among the most common adverse occurrences during mechanical ventilation. Severe alkalemia may precipitate cardiac arrhythmias or seizures. Unintentional respiratory alkalosis (pH >7.55 units) developed in 11% of ventilated patients in one series, and was associated with an increased overall mortality rate. Dyspnea, agitation, and pain are the most common causes in patients who do not suffer severe central nervous system dysfunction or chronic liver disease, and appropriate ventilator adjustment, combined with sedation as required, controls the alkalosis in most instances.
Renal and hepatic effects
Fluid retention and edema are common in patients who receive mechanical ventilation. Reductions in renal blood flow and impairment in renal function, especially with the use of PEEP, have been documented by a number of investigators, who postulate mechanisms that include elevation of serum antidiuretic hormone, excessive aldosterone effect, and (more recently) reduced levels of atrial natriuretic peptides.
Liver dysfunction is also fairly common in ventilated patients, which has led some investigators to conclude that positive-pressure ventilation causes hepatic impairment. A fall in portal blood flow has been documented with PEEP therapy, but the clinical importance of this is unclear. As with a number of the other adverse effects discussed here, it may be difficult to separate the effects of mechanical ventilation on renal and hepatic function from manifestations of the patient’s underlying disease process.
Dynamic Hyperinflation and Auto-PEEP
Pathogenesis
No concept is more important in understanding and managing mechanical ventilation in patients who have obstructive lung disease than that of dynamic hyperinflation. As mentioned above, COPD and asthma are characterized physiologically by hyperinflation and expiratory airflow limitation. When airway obstruction is severe, sufficient time may not be available to exhale completely a given tidal breath prior to the next inspiration. This means that that next breath begins at a higher lung volume. Over the course of several such breaths, the patient becomes progressively hyperinflated, and reaches a new FRC considerably higher than that at which the sequence began. Patients who have severe COPD may experience this phenomenon spontaneously during panic attacks or at times of increased dyspnea, but it can be especially serious during positive-pressure ventilation.
Clinical manifestations
As a result of the elastic recoil of the lung when distended above FRC (and of the chest wall at even larger lung volumes), dynamic hyperinflation is associated with positive end-expiratory alveolar pressure, or auto-PEEP (also called intrinsic PEEP, dynamic PEEP, or endogenous PEEP). The physiologic effects of auto-PEEP are the same as those of dialed-in (extrinsic) PEEP, and include decreased cardiac preload because of diminished venous return into the chest. The reduced cardiac output that results from the reduction in preload can lead to hypotension and, if severe, to pulseless electrical activity and cardiac arrest. Dynamic hyperinflation can also lead to local alveolar overdistention and rupture, followed by pneumothorax and other forms of extra-alveolar air (clinical barotrauma, see below).
Auto-PEEP is common in ventilated patients who have underlying COPD. Several other clinical circumstances suggest an increased likelihood of dynamic hyperinflation and auto-PEEP. Of special importance is the circumstance in which the patient appears to exert unusual effort to trigger the ventilator, or if airflow from the ventilator is not initiated with every inspiratory effort. Figure 7 illustrates why the latter occurs.
Fig 7
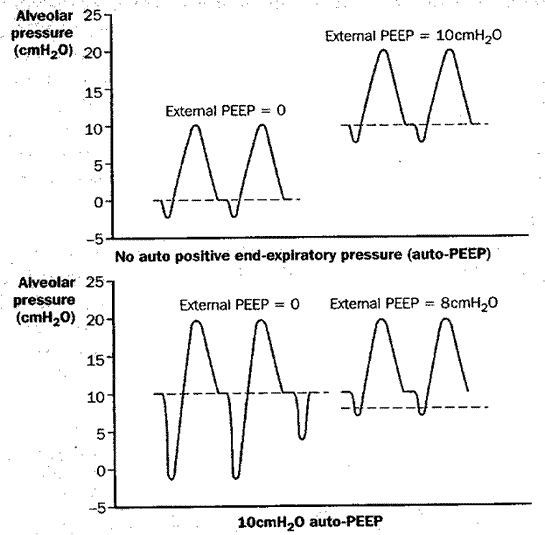
Figure 7: Impact of dynamic hyperinflation and auto-PEEP on a patient’s ability to trigger the ventilator, and the effect of adding external PEEP. Upper diagram: Triggering the ventilator in the absence of auto-PEEP. The addition of external PEEP does not affect the relative ease of initiating breaths from the ventilator. Lower diagram: Triggering the ventilator in the presence of 10 cm H2O of auto-PEEP. In the absence of external PEEP (left portion of each figure), in order to initiate inspiratory flow from the ventilator, the patient must generate enough ventilatory muscle contraction to overcome the 10 cm H2O of auto-PEEP in order to reduce tracheal pressure below zero. One occasion (lower left, 3rd breath attempt), insufficient force is generated to trigger the ventilator. When 8 cm H2O of external PEEP is added, the patient can trigger the ventilator much more easily, since much less ventilatory muscle contraction is needed to bring tracheal pressure down to the external PEEP level in order to initiate inspiratory flow.
Detection and measurement
Unlike externally applied PEEP, which is indicated on the ventilator’s pressure manometer, auto-PEEP cannot be detected unless a special maneuver is performed to allow pressures to equilibrate from the patient’s alveoli to the exhalation valve of the ventilator circuit at end expiration. At the author’s institution, respiratory practitioners who care for ventilated patients routinely check for the presence and magnitude of auto-PEEP (at least once each shift) in every patient who does not make spontaneous ventilatory efforts. When patients breathe spontaneously or attempt to trigger the ventilator, the end-expiratory occlusion technique cannot be used, and a different method such as stepwise addition of external PEEP must be used. Table 12 [21k PDF*] lists the different techniques available for detecting and quantitating auto-PEEP.
Prevention and management
The key to prevent the development of dynamic hyperinflation, and to reduce its severity when present, is to increase expiratory time. Dynamic hyperinflation occurs because insufficient time is available to complete lung emptying following each positive-pressure breath. Therefore, prevention and management hinge on measures to reduce airway obstruction, and to decrease the time spent on inspiration during each minute. Table 13 [15k PDF*] lists practical steps that can be taken to prevent auto-PEEP in ventilated patients, and to minimize it when present.
If the patient makes active inspiratory efforts, the addition of external PEEP decreases the muscular effort needed to trigger breaths from the ventilator, and thus decreases work of breathing and increases patient comfort. The added external PEEP should be at about 80% of the measured or estimated auto-PEEP level, as the latter is an average of various actual auto-PEEP levels in different lung regions, some of which are lower than the measured auto-PEEP. The level of auto-PEEP is checked frequently as the patient’s condition changes, with adjustments in external PEEP made accordingly.
Barotrauma
Pneumothorax, pneumomediastinum, subcutaneous emphysema, and other forms extra-alveolar air detected during mechanical ventilation are collectively termed ‘barotrauma.’ Although this term implies that excessive pressure is the cause, it is more likely that alveolar disruption results from overdistention (i.e., excessive peak inflating volume) rather than high pressure per se. Extra-alveolar air that first becomes evident during ventilatory support may also be unrelated to the ventilator.
Depending upon how much air enters the pulmonary interstitium and where the path of least resistance takes it, a variety of clinical manifestations can ensue. Pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema are rarely of physiologic significance and do not require specific treatment. However, pneumothorax is different in that the air collects in a space (the pleural cavity) from which it cannot naturally escape as pressure increases. Pneumothorax in a patient who receives positive-pressure ventilation can quickly progress to tension pneumothorax, which may rapidly cause cardiovascular collapse and death; for this reason, it must always be relieved promptly if ventilatory support cannot immediately be discontinued.
Table 14 [16k PDF*] outlines steps to minimize the risk of alveolar rupture and barotrauma during mechanical ventilation. These steps are especially important in high-risk patients, such as those who have obstructive or unevenly-distributed lung disease, pulmonary infection, and late-phase ARDS (e.g., beyond 1–2 weeks).
Ventilator-induced parenchymal lung injury, apart from clinically apparent barotrauma as discussed here, has been shown to affect both lung dysfunction and clinical outcomes in acute lung injury. Repetitive opening and closing of collapsed alveoli subjects them to high local pressures and can lead to activation of inflammatory mediators (so-called "biotrauma"). Similarly, overdistention of alveoli or lung regions can injure them without producing extra-alveolar air. Current evidence indicates that the prevention or reduction of ventilator-induced lung injury is an important reason for improved survival and other outcomes when patients with acute lung injury or ARDS are managed by the lung-protective ventilatory strategy discussed earlier.
Other complications
Several important complications associated with invasive mechanical ventilation are not discussed in this chapter. However, three important topics are briefly discussed here that are not generally included in reviews of ventilator complications.
‘Fighting the ventilator’ Agitation and respiratory distress that develop in a patient on a mechanical ventilator who has previously appeared comfortable represents an important clinical circumstance for which the clinician needs an organized approach. The patient should not automatically be sedated, but must instead be evaluated for several potentially life-threatening developments that can present in this fashion.
The ventilator is first disconnected from the patient, and the patient ventilated manually using 100% oxygen and a self-inflating bag. If this procedure relieves the patient’s distress, the problem lies proximal to the endotracheal tube, and the ventilator and its circuitry must be inspected or replaced. If agitation and respiratory distress persist, the airway are suctioned and a brief physical examination performed, noting recent trends in vital signs and any other new developments in the hours that preceded the onset of distress. A more detailed examination is required if the likely cause is not apparent, which must include a new set of measurements of arterial blood gases and a bedside chest radiograph. On rare occasions, if the patient appears in extremis and tension pneumothorax cannot be excluded at the bedside, empiric measures to drain the chest may be warranted.
Deteriorating oxygenation in the ventilated patient Deteriorating arterial oxygenation during mechanical ventilation, a common reason for ‘fighting the ventilator’, should initiate a systematic search for specific mechanisms and therapy rather than simply an increase in inspired oxygen fraction or level of PEEP. Possible causes for worsening oxygenation fall into several categories.
The problem could be with the ventilator and its circuitry. The patient’s primary disease process (e.g., pneumonia, ARDS) could be worsening, or a new medical problem may have appeared. Examples of the last include pneumothorax, acute lobar atelectasis, pulmonary edema from fluid overload, nosocomial pneumonia or sepsis, aspiration of gastric contents, retained secretions, and bronchospasm. A fall in cardiac output can also cause worsening oxygenation in a patient who has significant pulmonary venous admixture.
Interventions and procedures can also lead to a decline in oxygenation. Examples include the effects of airway suctioning, chest physical therapy, or even changes in body position, especially in patients affected by heterogeneously distributed pulmonary involvement. Bronchoscopy, thoracentesis, and hemodialysis can also lead to a decline in oxygenation. Finally, a number of drugs administered to patients undergoing mechanical ventilation can interfere with arterial oxygenation. Among these are vasodilators (which can decrease hypoxic vasoconstriction), b-blockers (which can depress cardiac output and induce bronchospasm), and bronchodilators (which can alter ventilation/perfusion ratios).
Effects of suboptimal ventilator management Some adverse effects of mechanical ventilation are iatrogenic. With increasing complexity of the ventilators and their modes, it becomes more likely that the physician, nurse, or respiratory therapist who adjusts the ventilator may not fully understand the consequences of a given adjustment. Table 15 [26k PDF*] lists several such iatrogenic problems, the clinical circumstances in which they typically occur, and steps to prevent or correct them.
*Software capable of displaying a PDF is required for viewing or printing. You may download a free copy of Adobe Reader from the Adobe website.
References
- Pierson DJ. Invasive mechanical ventilation. In Albert RK, Spiro SG, Jett JR, eds. Clinical respiratory medicine. London/Philadelphia, Saunders, 2nd edition, 2004:189-209
- MacIntyre NR, Cook DJ, Guyatt GH, eds. Evidence-based guidelines for weaning and discontinuing ventilatory support. American College of Chest Physicians, American Association for Respiratory Care, and American College of Critical Care Medicine. Chest. 2001 Dec;120(6 Suppl):375S-484S.
back to Page 1 | Page 2
*Software capable of displaying a PDF is required for viewing or printing. You may download a free copy of Adobe Reader from the Adobe website.
UW School of Medicine : School of Medicine Mission
Copyright and Disclaimer : Credits and Acknowledgements