


 |  |  |
|
Field Sampling Equipment Conductivity
Temperature Depth (CTD) A CTD (Conductivity, Temperature, Depth recorder) is
an instrument that is used to measure the pressure, temperature, conductivity (salinity),
dissolved oxygen, fluoresce, and transmissivity. The CTD is enclosed in a rectangular
stainless steel cage. On smaller boats, a Niskin bottle attached just above the
CTD was lowered into the water on a line using a pot puller winch and guided by
human hands. A Niskin bottle was used in order to collect water samples from
deeper stations. At the desired depth, the messenger was attached to the cable
and triggered the Niskin Bottle to close and collect a sample. Then the CTD and
Niskin Bottle were raised back to the surface where samples for dissolved
oxygen, chlorophyll, and nutrient samples could be extracted. The CTD model
that was used was a Seabird 19. |
 |  |  |
|
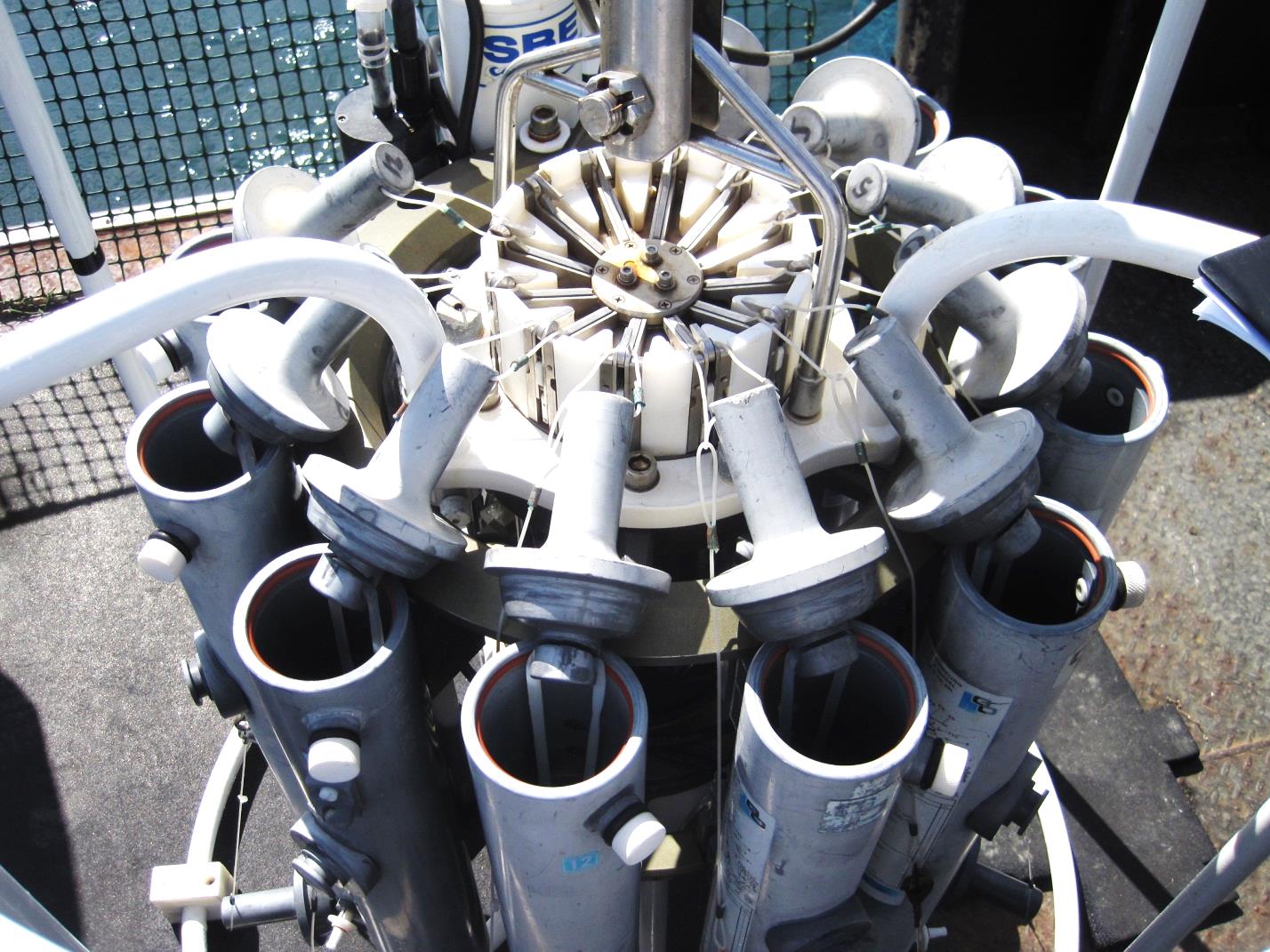
Niskin Bottle on a Rosette with a CTD On bigger boats, such as the R/V Centennial and R/V Barnes, a CTD with a rosette was used. The Rosette had 12 Niskin bottles surrounding a CTD. Both the rosette and Niskin bottles had a stainless steel cable connected to the boat's computer program system in real time. Each bottle was electronically tripped at selected depths. The big screen TV inside the boat showed a graph for the measurements of all the data as the CTD with the rosette were lowered into the water.  |
|
Thoreson Bottle The Thoreson bottle was used to collect surface and thermocline water samples. It works the same way as a Niskin bottle works except there is no copper messenger as a weight to send down the line. As an alternative, a quick jerking on the line tripped the bottle closed and trapped the water samples taken at that depth. It was very important to ensure that the bottle is properly secured open before it was lowering over the side of the vessel. It was essential to keep the Thoreson bottle under the water and keep oxygen out of the sample so it did not skew the dissolved oxygen analysis.  |
|
Discrete Water Samples Dissolved Oxygen |
 |  |
|
Chlorophyll |
 |
|
Nutrients One nutrient sample was collected at each depth. Samples were triple rinsed with collected water and filled 3/4 full to allow for freezing. |
 |
|
Phytoplankton Phytoplankton samples were collected using the phytoplankton 20 Ám net and the Niskin bottle. Net samples were collected by slowly dragging the phytoplankton net along the surface of the water column. Water trapped in the cod end of the net was collected for counting. These samples are qualitative and used only to estimate abundance of phytoplankton species by genus. Surface samples were collected from the Niskin bottle and collected in 125 mL jars, which were filled completely. These samples are quantitative due to the fixed volume of the sampling jars and were used to approximate phytoplankton populations in cells/L. |
 |
|
Zooplankton Zooplankton populations were collected using a 1 meter zooplankton 150 Ám net at station at only some of the stations. The vertical tow zooplankton net was plunged below the surface and is triggered by a messenger to close at a chosen depth. The net was dropped to 150 meter depth, towed back up to 50 meter, closed by the messenger, and brought on board. Zooplankton were collected in the cod end of the net and gathered into sampling jars. Counts were quantitative in order to approximate zooplankton population in cells/L. |
 |
 |
Sediment Sediments were collected at certain stations by using a Van Veen. This equipment was used after all the samples at the stations were collected. Van Veen had a spring loaded pin that triggered the grab when it touches the botton, then it was brought on board by pulling on the line attached to it. Once sediments were retrieved they were transferred to a labeled plastic bag, and placed on ice in a dark cooler. |
 |  |
|
Microplastics This method was used in order to collect plastic debris on the surface. It requires a specialized Manta net specifically designed and calibrated to sample the top 20 centimeters of surface water. It was towed behind the boat for 15 minutes, at an average speed of 3 knots. The Manta 330 Ám net is constructed as modified plankton net, allowing for micro-plastic particles to be collected in the cod end of the net. A flow meter within the net recorded the volume of water that passed through the net allowing for later calculations of micro-plastic concentrations per volume of seawater |
 |  |
|
Secchi Disk A 20 cm diameter secchi disk was lowered in the water at each station until it was no longer visible. The last depth at which it was visible is recorded as the depth of water visibility for each station. |
 |
Laboratory Procedures for Collected Water Samples Dissolved
Oxygen Oxygen samples were processed using the procedure for the Carpenter modification of the Winkler Method (Carpenter, 1965). A Dosimat was used for the titrations. Three standards were run, and at least 2 out of 3 agreed within 0.001 mL. A blank was prepared to determine a blank correction factor. During titration, 1 mL H2SO4 was added to each sample and the sample was titrated with thiosulfate. When the sample became pale yellow, 1mL starch indicator was added and each sample was titrated to the endpoint Procedure: Lou Codispoti Advice on Determination of Dissolved Oxygen in Seawater
https://fortress.wa.gov/ecy/publications/publications/0610091.pdf |
|
Chlorophyll Chlorophyll samples were processed using the UWT Chlorophyll Procedure (EPA Method 445). For analysis, samples were vacuum-pumped through a MgCO3-wetted Whatman GF/F 25mm glass fiber filter. This filter was then placed in 10 ml of 90% acetone and placed in a sonicator for seven minutes at 50%. After a further ten minutes, each sample was centrifuged at 3,700 RPM for five minutes. The supernatant was then run through two fluorometers to measure concentration of chlorophyll and phaeopigment. Samples were processed using both a Turner Model TD700 Fluorometer (which was the “broken” machine), and a Turner Trilogy Fluorometer. The results of both fluormeters were also compared to each other to evaluate the precision of the equipment.Procedure: Chlorophyll Procedure |
|
Phytoplankton Phytoplankton counts were conducted for both net and surface locations. Surface samples were fixed after collection and net samples were not. Nets were qualitatively evaluated live. Surface and thermocline samples were fixed using the Estuaries—Phytoplankton Protocol (Huber, 2012a). After 24 hours, the excess water in these samples was decanted above the 10mL line. Sedgewick-Rafter counting chambers were used to count phytoplankton samples. Counting was executed using the Estuaries Phytoplankton Protocol (Huber, 2012). In surface a sample, full transects were counted until 100 organisms were enumerated. For net samples, only one transect was counted for each sample and relative abundance of each genus found was assessed. The depth of transect was determined as 1mm, length 50mm, and width 2mm. On 100X magnification, the “ticks” of the ocular scale equated to 10ticks/mm and the volume per transect equated to 0.1mm3 ((D x L x W) x (1 mL/ 1000mm3). To calculate the concentration use the following equation:(cells counted / slide volume) x (1 / concentration factor) x (1000 mL / 1 L) = # cells/L Procedure: Fixing Plankton Sample |
|
Zooplankton Sedgewick-Rafter counting chambers were used to count zooplankton samples. One transect was counted for each sample and relative abundance of each genus found was assessed. The depth of transect was determined as 1mm, length 50mm, and width 2mm. On 100X magnification, the “ticks” of the ocular scale equated to 10ticks/mm and the volume per transect equated to 0.1mm3 (D x L x W) x (1 mL/ 1000mm3). To calculate the concentration use the following equation:(cells counted / slide volume) x (1 / concentration factor) x (1000 mL / 1 L) = # cells/L
|
|
Nutrients Nutrients samples were submitted to University of Washington, Seattle for analysis.Please visit UW Seattle School of Oceanography for more information. |
|
Sediments Collected sediment samples were taken to the lab, and sorted for grain size by using the Coulter LS 200 Particle Size Analyzer. The total organic content (TOC) was calculated in the laboratory by loss on ignition. Particle Size Analysis and TOC Protocol were followed in the sediment analysis (Huber, 2012b). Procedure: Particle Size Analysis Protocol |
|
Microplastics Microplastics samples will be analyzed by trained undergraduate students using laboratory methods for the analysis of microplastics in the marine environment (Baker et al., 2011) and reported on at a later time. Procedure: Microplastics Sample SOP |
| Banner Pictures from left to right: http://www.travelphotoadventures.com/wp-content/uploads/2012/07/44-Puget-Sound-Sunset-1.jpg http://bikestylespokane.com/wp-content/uploads/2012/06/Seattle-Skyline-at-Night-with-Reflections-in-Water.jpg http://interfacelift.com/wallpaper/D0249fa9/02102_elliottbayferry_1600x1200.jpg |